��Ŀ����
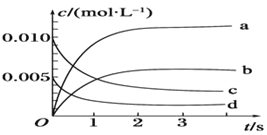
����Ŀ��H2C2O4Ϊ��Ԫ���ᡣ20��ʱ������һ��c��H2C2O4��+ c��HC2O4-��+ c��C2O42-��=0.100 mol��L-1��H2C2O4��NaOH�����Һ����Һ�в����������ʵ���Ũ����pH�ı仯��������ͼ��ʾ������ָ����Һ���������ʵ���Ũ�ȹ�ϵһ����ȷ������ ��

A��pH=2.5����Һ�У�c��H2C2O4��+c��C2O42-����c��HC2O4-��
B��c��Na+����0.100 mol��L-1����Һ�У�c��H+��+c��H2C2O4����c��OH-��+c��C2O42-��
C��c��HC2O4-����c��C2O42-������Һ�У�c��Na+����0.100 mol��L-1+c��HC2O4-��
D��pH=7����Һ�У�c��Na+����2c��C2O42-��
���𰸡�BD
��������
���������A������ͼ��֪pH��2.5����Һ�У�c��H2C2O4��+ c��C2O42-���� c��HC2O4-����A����B�����������غ�͵���غ����c��Na+����0.100 mol��L-1����Һ�У�c��H+��+c��H2C2O4����c��OH-��+ c��C2O42-����B��ȷ��C���ؼ�ͼ���֪c��HC2O4-����c��C2O42-������Һ�����ԣ���ҺΪ�����ʵ���Ũ�ȵIJ������ƺͲ����ƵĻ����Һ������Һ�У�c��Na+����0.100 mol��L-1+ c��HC2O4-����C����D����ͼ��֪pH��7����ҺΪ��������Һ�������ˮ�⣬c��Na+����2c��C2O42-����D��ȷ����ѡBD��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�