��Ŀ����
�����仯����������������й㷺Ӧ�á���ش��������⣺
(1)������(FeS2)�����������ұ����������Ҫԭ�ϡ�����һ����ӦΪ3FeS2��8O2 6SO2��Fe3O4����3 mol FeS2�μӷ�Ӧ��ת��________ mol���ӡ�
6SO2��Fe3O4����3 mol FeS2�μӷ�Ӧ��ת��________ mol���ӡ�
(2)�Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ________________���Ӹ�ʴ��Һ���յõ�����ͭ������Ҫ���Լ���__________________________��
(3)���������ƣ�������Ҳ������ˮ������ʹ��ʱ����������������ʹ���Է�ˮ�е������������ȥ����ԭ����____________________________
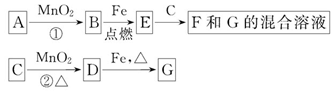
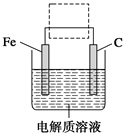
(4)�����ĵ绯ѧ��ʴԭ����ͼ��ʾ������ͼ�����ļ��ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ������ͼ�����߿��������ģ����ü�ͷ���������������
(1)������(FeS2)�����������ұ����������Ҫԭ�ϡ�����һ����ӦΪ3FeS2��8O2
 6SO2��Fe3O4����3 mol FeS2�μӷ�Ӧ��ת��________ mol���ӡ�
6SO2��Fe3O4����3 mol FeS2�μӷ�Ӧ��ת��________ mol���ӡ�(2)�Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ________________���Ӹ�ʴ��Һ���յõ�����ͭ������Ҫ���Լ���__________________________��
(3)���������ƣ�������Ҳ������ˮ������ʹ��ʱ����������������ʹ���Է�ˮ�е������������ȥ����ԭ����____________________________
(4)�����ĵ绯ѧ��ʴԭ����ͼ��ʾ������ͼ�����ļ��ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ������ͼ�����߿��������ģ����ü�ͷ���������������

(1)32
(2)2Fe3����Cu=2Fe2����Cu2�������ۡ�ϡ����(ϡ����)
(3)���Է�ˮ����Fe3����ˮ�⣬ʹ�䲻���������������õ�Fe(OH)3����
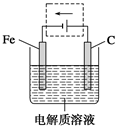
(4)
(2)2Fe3����Cu=2Fe2����Cu2�������ۡ�ϡ����(ϡ����)
(3)���Է�ˮ����Fe3����ˮ�⣬ʹ�䲻���������������õ�Fe(OH)3����
(4)

(1)��Ӧ��FeS2�е�Fe��Sʧ���ӷ���������Ӧ��O2�õ��ӷ�����ԭ��Ӧ��8 mol O2�õ��ĵ�����Ϊ32 mol����Ϊ�÷�Ӧ��ת�Ƶĵ���������
(2)����ͭ�Ĺ����У�Fe3����Cu����ΪCu2��������������ͭ�û�������Ϊ��ȥ���������ۿ��Լ�������ϡ�����ϡ���ᡣ
(3)Fe3��ˮ�����Һ�����ԣ������Է�ˮ��H��������Fe3����ˮ�⡣
(4)�绯ѧ������������ԭ���ԭ����Ҳ�����ǵ���ԭ������ʹ������ӵ�Դ�ĸ�������������������Ч��ֹ������ʴ��
(2)����ͭ�Ĺ����У�Fe3����Cu����ΪCu2��������������ͭ�û�������Ϊ��ȥ���������ۿ��Լ�������ϡ�����ϡ���ᡣ
(3)Fe3��ˮ�����Һ�����ԣ������Է�ˮ��H��������Fe3����ˮ�⡣
(4)�绯ѧ������������ԭ���ԭ����Ҳ�����ǵ���ԭ������ʹ������ӵ�Դ�ĸ�������������������Ч��ֹ������ʴ��

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
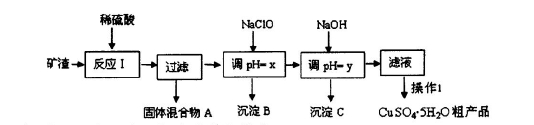
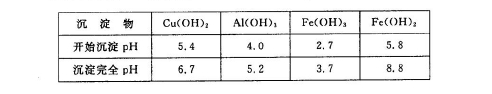


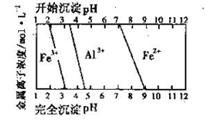

 FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______��
FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______��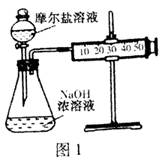
 Cu2++Cu�����ڷ�Ӧ�¶Ȳ�ͬ,��������ԭ����ͭʱ,���ܲ���Cu��Cu2O,���߶��Ǻ�ɫ���塣һͬѧ��ij����������ԭ����ͭʵ�����õĺ�ɫ�������������֤,ʵ�������ʵ�������¼����:
Cu2++Cu�����ڷ�Ӧ�¶Ȳ�ͬ,��������ԭ����ͭʱ,���ܲ���Cu��Cu2O,���߶��Ǻ�ɫ���塣һͬѧ��ij����������ԭ����ͭʵ�����õĺ�ɫ�������������֤,ʵ�������ʵ�������¼����: