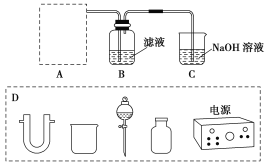
��Ŀ����
����Ŀ��Ϊ�ⶨij�л�������A�Ľṹ����������ʵ��.
��.����ʽ�IJⶨ
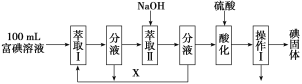
��1�����л���A �����������г��ȼ�գ�ʵ����.����5.4gˮ��8.8g������̼����������6.72L����״���£���������ʵĻ�ѧʽ��________________.
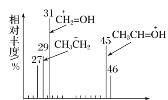
��2���������Dzⶨ���л����������Է����������õ���ͼ��ʾ����ͼ��������Է�������Ϊ ___________ .�����ʵķ���ʽΪ_____________ .
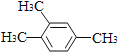
��3�������л�������ijɼ��ص㣬Ԥ��A�Ŀ��ܽṹ��ʽ��д���ṹ��ʽ__________.
��.�ṹʽ��ȷ��
��4���˴Ź��������ܶ��л�����������в�ͬλ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ���źţ������ݷ�ֵ���źţ�����ȷ����������ԭ�ӵ��������Ŀ�����ⶨ���л���A�ĺ˴Ź�������ʾ��ͼ��ͼ����A�й��������� ____________.

���𰸡�C2H6O 46 C2H6O CH3CH2OH��H3C-O-CH3 �ǻ�
��������
��.(1)����ԭ���غ���㡣
(2)������������ʾ����ͼ������Է�������Ϊ46������ʽΪC2H6O��
(3)�����л�������ijɼ��ص㣬�����в��������ͼ���
��.(4)���ݺ˴Ź������ף������й�����3����ԭ�ӣ����л���A�Ľṹ��ʽΪCH3CH2OH��
��.(1)5.4gˮ�����ʵ���Ϊ0.3mol��8.8g������̼�����ʵ���Ϊ0.2mol����������6.72L����״���£������ʵ���Ϊ0.3mol������ԭ���غ㣬�л���A����0.2mol��C��0.6mol��H��0.1mol��O����C��H��O�����������Ϊ2��6��1������ʽΪC2H6O��
(2)������������ʾ����ͼ������Է�������Ϊ46������ʽΪC2H6O��
(3)�����л�������ijɼ��ص㣬�����в��������ͼ������ܵĽṹ��ʽΪCH3CH2OH��H3C-O-CH3��
��.(4)���ݺ˴Ź������ף������й�����3����ԭ�ӣ����л���A�Ľṹ��ʽΪCH3CH2OH�����еĹ�����Ϊ�ǻ���

 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�����Ŀ��д������������Ȼ�ѧ����ʽ��
(1)0.5molCH4��ȫȼ������CO2��Һ̬ˮʱ���ų�445kJ ��������д��CH4ȼ�յ��Ȼ�ѧ����ʽ__________��
(2)ͨ�����ǰѲ�1molij��ѧ�����յ��������ɸû�ѧ���ļ��ܡ��±���һЩ��ѧ���ļ��ܡ�
��ѧ�� | C��H | C��F | H��F | F��F |
����kJ/mol | 414 | 489 | 565 | 155 |
���ݼ������ݹ������з�Ӧ��CH4(g) + 4F2(g)��CF4(g) + 4HF(g)�ķ�Ӧ�ȡ�HΪ_____��
(3)1840����ʿ�Ļ�ѧ�Ҹ�˹(Hess)���ܽ����ʵ����ʵ(�Ȼ�ѧʵ������)�Ļ��������������ѹ���������µ����⻯ѧ��Ӧ���ڲ���������ʱ��������һ����ɵĻ��Ǽ�����ɵģ�����ЧӦ������ͬ��(��Ӧ�ȵ���ֵ���)����
��֪��Fe2O3(s)��3CO(g)��2Fe(s)��3CO2(g) ��H1
3Fe2O3(s)��CO(g)��2Fe3O4(s)��CO2(g) ��H2
Fe3O4(s)��CO(g)��3FeO(s)��CO2(g) ��H3
��д��CO��ԭFeO���Ȼ�ѧ����ʽ��______��
(4)��101KP�£�1g������ȫȼ������Һ̬ˮ�ų�142.9kJ����������ش��������⣺
��������ȼ����Ϊ__________��
������ȼ���ȵ��Ȼ�ѧ����ʽΪ________��
(5)���ܵĴ洢���������õ�ǰ�ᣬ��ѧ���о���һ�ִ���Ͻ�Mg2Ni����֪��
Mg(s)��H2(g)=MgH2(s) ��H1����74.5kJ��mol��1��
Mg2Ni(s)��2H2(g)=Mg2NiH4(s) ��H2��
Mg2Ni(s)��2MgH2(s)=2Mg(s)��Mg2NiH4(s) ��H3��+84.6kJ��mol��1��
����H2��_______kJ��mol��1��
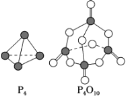
(6)�����������ɷ������·�Ӧ��P4��5O2=P4O10����֪�������л�ѧ����Ҫ���յ������ֱ�ΪP��P a kJ��mol��1��P��O b kJ��mol��1��P=O c kJ��mol��1��O=O d kJ��mol��1������ͼʾ�ķ��ӽṹ���й����ݹ���÷�Ӧ����HΪ_____��
(7)ͬ���������ת���ķ�Ӧ���൱С����ת�����ʽ�������ʱ���ܲ���ȫ���ⶨ��Ӧ�Ⱥ����ѡ����ڿɸ��ݸ�˹����������ܻ�ѧ������һ����ɻ�ּ�����ɣ�����ܹ��̵���ЧӦ����ͬ�����۵������㷴Ӧ�ȡ���֪��
��P4(���ף�s)��5O2(g)=P4O10(s) ��H1=��2 983.2 kJ��mol��1
��P(���ף�s)��5/4O2(g)=1/4P4O10(s) ��H2=��738.5 kJ��mol��1
��ͬ״���£������ϵ͵���________�������ȶ��ԱȺ���________(������������С��)��