��Ŀ����
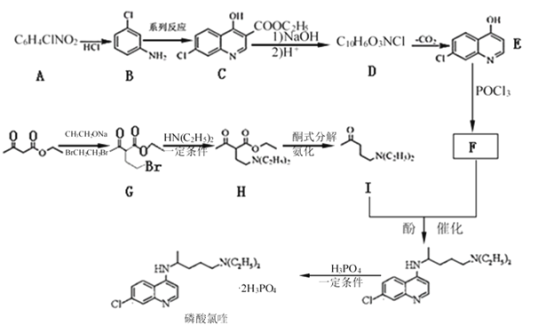
����Ŀ�����ͱ�������������ҩ��,��ϳ�·������:
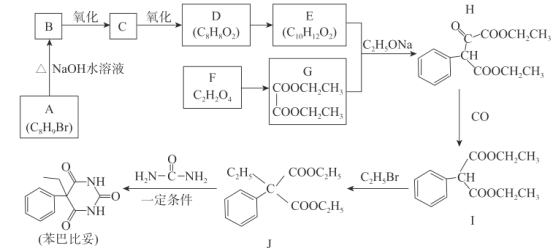
��ش���������:
(1)G�Ļ�ѧ����Ϊ_________,C�ķ���ʽΪ__________��
(2)F+X��G+ H2O,X�Ľṹ��ʽΪ____________��
(3)H�к��еĹ���������Ϊ__________,I��J�ķ�Ӧ����Ϊ______________��
(4)ͬʱ��������������E��ͬ���칹�干��______�֡�
�ٺ��б����ұ�����������ȡ����
���ܷ���ˮ�ⷴӦ���ܷ���������Ӧ
(5)������J�����ͱ��Ļ�ѧ����ʽΪ________________________________��
(6)��1,3-����ϩ���Ҵ�������(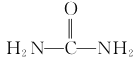 )Ϊԭ�Ϻϳ�
)Ϊԭ�Ϻϳ�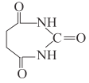 �������Լ���ѡ,��д������:_____________________________
�������Լ���ѡ,��д������:_____________________________
���𰸡��Ҷ�������� C8H8O CH3CH2OH �������ʻ� ȡ����Ӧ 15 ![]() +
+ +2C2H5OH CH2=CH��CH=CH2
+2C2H5OH CH2=CH��CH=CH2![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br ![]() HOCH2CH2CH2CH2OH
HOCH2CH2CH2CH2OH![]() HOOCCH2CH2COOH
HOOCCH2CH2COOH![]() C2H5OOCCH2CH2COOC2H5
C2H5OOCCH2CH2COOC2H5 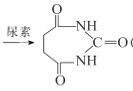
��������
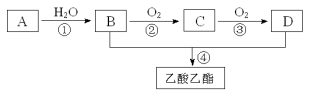
A����ʽΪC8H9Br������H�ṹ��ʽ��A��Ӧ���б�����A����������ˮ��Һ����B������±������ˮ�⣬�ǻ�ȡ����Br��λ�ã�B������C������D�����Ǵ���������ȩ��ȩ���������ᣬ��A�Ľṹ��ʽΪ![]() ��B�Ľṹ��ʽΪ
��B�Ľṹ��ʽΪ![]() ��C�Ľṹ��ʽΪ
��C�Ľṹ��ʽΪ![]() ��D�Ľṹ��ʽΪ
��D�Ľṹ��ʽΪ![]() ���ݴ˷�����
���ݴ˷�����
(1)����G�ṹ��ʽ��G�Ļ�ѧ����Ϊ�Ҷ����������A����ʽΪC8H9Br������H�ṹ��ʽ��A��Ӧ���б�����A����������ˮ��Һ����B������±������ˮ�⣬�ǻ�ȡ����Br��λ�ã�B������C������D�����Ǵ���������ȩ��ȩ���������ᣬ��A�Ľṹ��ʽΪ![]() ��B�Ľṹ��ʽΪ
��B�Ľṹ��ʽΪ![]() ��C�Ľṹ��ʽΪ
��C�Ľṹ��ʽΪ![]() ��D�Ľṹ��ʽΪ
��D�Ľṹ��ʽΪ![]() ����C�ķ���ʽC8H8O��
����C�ķ���ʽC8H8O��
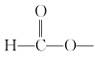
(2)����F�ķ���ʽ���Լ�G�Ľṹ��ʽ��F��X����������Ӧ����FΪ�Ҷ��ᣬX�ṹ��ʽΪCH3CH2OH��
(3)����H�Ľṹ��ʽ�����еĹ��������������ʻ����Ա�I��J�ṹ��ʽ��C2H5Br�еģ�C2H5ȡ��I�С�CH���ϵ�H����I��JΪȡ����Ӧ��
(4)�ٺ��б�����������������ȡ������������ȡ����λ��Ϊ�ڡ��䡢�ԣ����ܷ���ˮ�ⷴӦ���ܷ���������Ӧ������E�ķ���ʽ����˸����ʽṹ���С� ��������������
�������������� �뱽��֮����������һ��ȡ����Ϊ��CH2CH2CH3����CH(CH3)3��
�뱽��֮����������һ��ȡ����Ϊ��CH2CH2CH3����CH(CH3)3�� ͬһ��Cԭ���뱽����������һ��ȡ����Ϊ��CH2CH3����
ͬһ��Cԭ���뱽����������һ��ȡ����Ϊ��CH2CH3����![]() �뱽��֮����������һ��ȡ����Ϊ��CH3����
�뱽��֮����������һ��ȡ����Ϊ��CH3����![]() �뱽��֮����������һ��ȡ����Ϊ��CH3����������������Ҫ���ͬ���칹���������3��5=15�֣�
�뱽��֮����������һ��ȡ����Ϊ��CH3����������������Ҫ���ͬ���칹���������3��5=15�֣�
(5)J�����ͱ�����ȡ����Ӧ����Ӧ����ʽΪ![]() +
+ +2C2H5OH��
+2C2H5OH��
(6)���ݺϳ����ʣ��Լ�J�����ͱ��ף���Ҫ�����ʳ������⣬����ҪC2H5OOCCH2CH2COOC2H5���������1��3������ϩ��Br2��CCl4��Һ����1��4���ӳɣ��õ�CH2BrCH=CHCH2Br��Ȼ���������������ӳɷ�Ӧ���õ�CH2BrCH2CH2CH2Br����NaOH��ˮ��Һ�з���ˮ�ⷴӦ���õ�HOCH2CH2CH2CH2OH������������HOOCCH2CH2COOH�����Ҵ�����������Ӧ���ϳ�·��ΪCH2=CH��CH=CH2![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br![]() CH2BrCH2CH2CH2Br
CH2BrCH2CH2CH2Br ![]() HOCH2CH2CH2CH2OH
HOCH2CH2CH2CH2OH![]() HOOCCH2CH2COOH
HOOCCH2CH2COOH![]() C2H5OOCCH2CH2COOC2H5
C2H5OOCCH2CH2COOC2H5  ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�