��Ŀ����
����Ŀ����1����Ҫ��д������ʽ��
��HNO3(���뷽��ʽ) _____________��
��Fe2(SO4)3(���뷽��ʽ) ___________��
�������ƺ��Ȼ�����Һ��Ӧ(���ӷ���ʽ) ______________��
�ܶ�����̼ͨ����������������Һ(���ӷ���ʽ)_________________��
��2������Ϊ��ѧ��ѧ�г����ļ������ʣ��ٶ�����̼��������KCl����NaHSO4���塡��ͭ����ϡ���ᡡ����ʯ��ˮ���������ڵ���ʵ���_________�����ڷǵ���ʵ���________(�����)��
���𰸡�![]()
![]()
![]()
![]() �ڢ� ��
�ڢ� ��
��������
����ʵĶ�������ˮ��Һ������״̬���ܵ���Ļ�����ҵ����ԭ������Ϊ�������ĵ��룬����ʺͷǵ���ʶ������ǻ�������ʺͻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ��ݴ˷�����
(1)��HNO3�ĵ��뷽��ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
��Fe2(SO4)3�ĵ��뷽��ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
�������ƺ��Ȼ�����Һ��Ӧ�����ӷ���ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
�ܶ�����̼ͨ����������������Һ�����ӷ���ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
(2)�ݷ�����֪�����Ƿǵ���ʣ��ڢ��ǵ���ʣ��ܢݢȲ��ǵ����Ҳ���Ƿǵ���ʣ�
�ʴ�Ϊ���ڢۣ��٣�

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�����Ŀ��ij��ȤС���ö�п��Ƥ�����������Ʊ���ˮ������п��ZnSO4��7H2O��
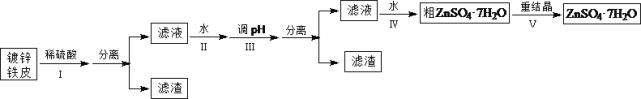
�����Ϣ���£��ٽ��������γ�����������������pH��Χ��
�������� | pH | |
��ʼ���� | ��ȫ���� | |
Fe3+ | 1.5 | 2.8 |
Fe2+ | 5.5 | 8.3 |
Zn2+ | 5.4 | 8.2 |
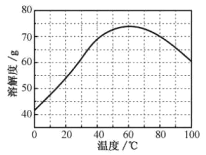
��ZnSO4���ܽ�ȣ�������100gˮ���ܽ�����������¶ȱ仯���ߡ�
��ش�
(1)�ٶ�п��Ƥ�ϵ����ۿ���Na2CO3��Һȥ����������_______________________________���ڲ���������ж϶�п����ȫ��Ӧ��ʵ��������_______________________________��
(2)�������������H2O2��������_______________________________��
(3)������ʵ�pH��Χ��_______________________________��
(4)���������Ҫ�õ���������������a.��������Һ���־�Ĥ��b.��60�������ܼ���c.��ȴ�����£�d.��100�������ܼ���e.���ˡ������������������ȷ˳��___________________���������ظ�ʹ�ã���
(5)�������ijͬѧ���ò�ͬ���·�ʽ������ȴ�ᾧ�����ZnSO4��7H2O���������С�ֲ���ͼ1��ʾ�����ݸ�ʵ������Ϊ�˵õ�������С��Ծ�һ�Ľϴ�������ѡ��_________��ʽ������ȴ�ᾧ��
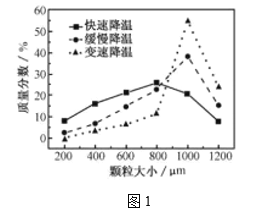
A.���ٽ��� B. �������� C.���ٽ���
(6)ZnSO4��7H2O��Ʒ�Ĵ��ȿ�����λ�ζ����ⶨ��
�� ���й��ڵζ���������ȷ����________________��
A.ͼ2�У�Ӧ����ʿ��Ϳ��������a�˺��������ڵ�c��

B.�ζ�ǰ����ƿ�͵ζ��ܾ����ñ���Һ��ϴ
C.������Һװ��ζ���ʱ��Ӧ�����ձ���©���Ȳ�������ת��
D.�ζ�ʱ��ͨ�������ֿ��������μ���Һ������ҡ����ƿ��ʹ��Һ��ͬһ������ת
E.�ζ�ǰ�ζ��ܼ����������ݣ��ζ�������������ݣ����õ������ʵ�����ĵ�С
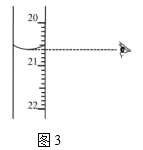
��ͼ3����ʾ�ζ��յ�ʱ�Ķ�����_____________mL��