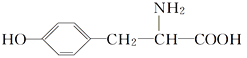��Ŀ����
����Ŀ��ij��ȤС���ö�п��Ƥ�����������Ʊ���ˮ������п��ZnSO4��7H2O��
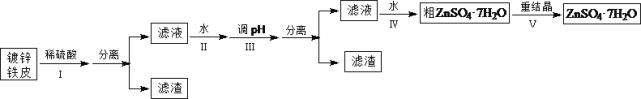
�����Ϣ���£��ٽ��������γ�����������������pH��Χ��
�������� | pH | |
��ʼ���� | ��ȫ���� | |
Fe3+ | 1.5 | 2.8 |
Fe2+ | 5.5 | 8.3 |
Zn2+ | 5.4 | 8.2 |
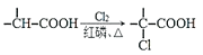
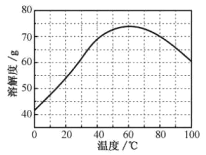
��ZnSO4���ܽ�ȣ�������100gˮ���ܽ�����������¶ȱ仯���ߡ�
��ش�
(1)�ٶ�п��Ƥ�ϵ����ۿ���Na2CO3��Һȥ����������_______________________________���ڲ���������ж϶�п����ȫ��Ӧ��ʵ��������_______________________________��
(2)�������������H2O2��������_______________________________��
(3)������ʵ�pH��Χ��_______________________________��
(4)���������Ҫ�õ���������������a.��������Һ���־�Ĥ��b.��60�������ܼ���c.��ȴ�����£�d.��100�������ܼ���e.���ˡ������������������ȷ˳��___________________���������ظ�ʹ�ã���
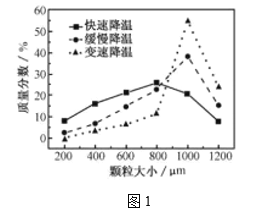
(5)�������ijͬѧ���ò�ͬ���·�ʽ������ȴ�ᾧ�����ZnSO4��7H2O���������С�ֲ���ͼ1��ʾ�����ݸ�ʵ������Ϊ�˵õ�������С��Ծ�һ�Ľϴ�������ѡ��_________��ʽ������ȴ�ᾧ��
A.���ٽ��� B. �������� C.���ٽ���
(6)ZnSO4��7H2O��Ʒ�Ĵ��ȿ�����λ�ζ����ⶨ��
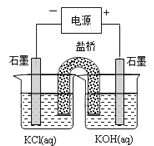
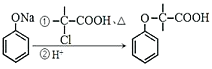
�� ���й��ڵζ���������ȷ����________________��
A.ͼ2�У�Ӧ����ʿ��Ϳ��������a�˺��������ڵ�c��

B.�ζ�ǰ����ƿ�͵ζ��ܾ����ñ���Һ��ϴ
C.������Һװ��ζ���ʱ��Ӧ�����ձ���©���Ȳ�������ת��
D.�ζ�ʱ��ͨ�������ֿ��������μ���Һ������ҡ����ƿ��ʹ��Һ��ͬһ������ת
E.�ζ�ǰ�ζ��ܼ����������ݣ��ζ�������������ݣ����õ������ʵ�����ĵ�С
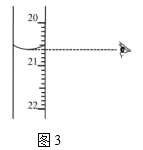
��ͼ3����ʾ�ζ��յ�ʱ�Ķ�����_____________mL��
���𰸡�Na2CO3ˮ�⣬��Һ�ʼ��ԣ���ʹ��֬ˮ�⣻ �������ݵ������������� ʹFe2+������ת��ΪFe3+��H2O2�ֽ⣨Fe3+�����ٷֽ⣩ 2.8~5.4 dabace C ADE 20.60
��������
��1���١�̼����ˮ���Ǽ��ԣ��ܹ�ʹ����ˮ�⣬�ﵽ��ȥ�������۵�Ч�����ڡ�п�㡢���㼰ϡ����ṹ��ԭ��أ���Ӧ���ʿ죬��п����ȫ�ܽ��ԭ��ؽṹ��ʧ����Ӧ�����½����������ݵ����ʼ�����
��2��ʹFe2+ת��ΪFe3+�����γɳ������룬������Fe3+��ٽ�H2O2�ķֽ⣬����Ҫ���������H2O2����ΪʹFe2+������ת��ΪFe3+��H2O2�ֽ⣨Fe3+�����ٷֽ⣩��
��3��Ҫʹ�������ӷ��룬��ҪʹFe3+��ȫ�������ֲ�ʹZn2+�����������pHΪ2.8![]() pH<5.4��
pH<5.4��
��4��Ҫ����Һ�еõ��ֲ�Ʒ�����ȼ���������ֱ�����־�Ĥ���ﵽ����״̬����ȥ�����ܼ�����ͼ��֪��60��ʱ��Ʒ���ܽ����ʽ�����60���������ܼ��������־�Ĥ���ﵽ����״̬��������ȴ�����£�ʹ��Ʒ�����������ˣ�ʹ��Һ���룬�õ��ֲ�Ʒ���ʲ�������ȷ˳��Ϊdabace��
��5�����ͼ���֪�ڱ��ٽ��µ�����µõ��Ŀ����ϴ�ѡC��
��6����A����ͿĨ������ʱ���ܽ�������С��ס����ͿĨ������a�˺��������ڵ�c�ˣ�ѡ��A��ȷ��B���ζ�ǰ��ƿ�����ñ�Һ��ϴ����ʹ�ⶨ�������ƫ�ѡ��B����C����Һװ��ζ���ʱֱ�ӵ��룬�������������������ܻ������Ⱦ�Լ���������ѡ��C������D���ζ�ʱ�������ֿ��������μ���Һ������ҡ����ƿ��ʹ��Һ��ͬһ������ת��ѡ��D��ȷ��E���ζ�ǰ�����ݡ��ζ���������ݣ��μӵ���Һ��ʵ�����ĵ����ҪС��ѡ��E��ȷ����ѡADE���ڵζ��ܵĶ����������£����Ҿ�ȷ��С�������λ������Ϊ20.60 mL��

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�