��Ŀ����
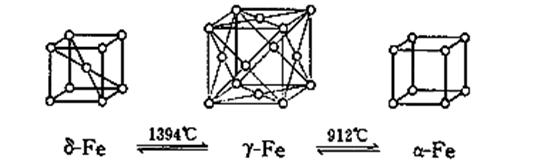
��15�֣�������BN����һ����Ҫ�Ĺ����մɲ��ϡ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��
��ش��������⣺
��1����B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������ ��
��
��2����̬Bԭ�ӵĵ����Ų�ͼΪ �� BN��BԪ�صĻ��ϼ�Ϊ ��
��3����BF3�����У�F��B��F�ļ����� ��Bԭ�ӵ��ӻ��������Ϊ ��
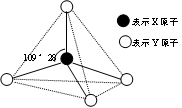
��4����֪�� BF3 + NaF��������= NaBF4��������NaBF4���еĻ�ѧ������Ϊ ��BF4����F��B��F�ļ����� ��Bԭ�ӵ��ӻ��������Ϊ ��BF4�������幹��Ϊ ��
��5������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��������Ϊ �����������Ϊ ����1 mol BN���������������к�B-N�ĸ���Ϊ NA��

��ش��������⣺
��1����B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������ ��
��
��2����̬Bԭ�ӵĵ����Ų�ͼΪ �� BN��BԪ�صĻ��ϼ�Ϊ ��
��3����BF3�����У�F��B��F�ļ����� ��Bԭ�ӵ��ӻ��������Ϊ ��
��4����֪�� BF3 + NaF��������= NaBF4��������NaBF4���еĻ�ѧ������Ϊ ��BF4����F��B��F�ļ����� ��Bԭ�ӵ��ӻ��������Ϊ ��BF4�������幹��Ϊ ��
��5������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��������Ϊ �����������Ϊ ����1 mol BN���������������к�B-N�ĸ���Ϊ NA��
1����4�֣�B2O3+3CaF2+3H2SO4 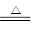 2BF3��+3CaSO4 +3H2O ��
2BF3��+3CaSO4 +3H2O ��
B2O3+2NH3 2BN+3H2O��
2BN+3H2O��
��2����2�֣�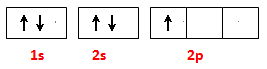 �� +3
�� +3
��3����2�֣�1200 �� sp2��
��4����4�֣����Ӽ������Թ��ۼ�����λ�� �� 109028�� �� sp3 ���������壻
��5����3 �֣� ���Թ��ۼ� �����»��� �� 3��
 2BF3��+3CaSO4 +3H2O ��
2BF3��+3CaSO4 +3H2O ��B2O3+2NH3
 2BN+3H2O��
2BN+3H2O����2����2�֣�
 �� +3
�� +3 ��3����2�֣�1200 �� sp2��
��4����4�֣����Ӽ������Թ��ۼ�����λ�� �� 109028�� �� sp3 ���������壻
��5����3 �֣� ���Թ��ۼ� �����»��� �� 3��
��1�����ݷ�Ӧ����������֪������ʽ�ֱ���
B2O3+3CaF2+3H2SO4 2BF3��+3CaSO4 +3H2O��B2O3+2NH3
2BF3��+3CaSO4 +3H2O��B2O3+2NH3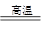 2BN+3H2O��
2BN+3H2O��
��2�����ݹ���ԭ����д����̬Bԭ�ӵĵ����Ų�ͼ����Ԫ���ǵڢ�A����ͼ��ǣ�3�ۣ�����BԪ�صĻ��ϼ��ǣ�3�ۡ�
��3����BF3�����У�����ԭ��Bԭ��û�й¶Ե��ӣ�������ṹ��ƽ�������Σ�������120�㡣����Bԭ�ӵ��ӻ��������Ϊsp2�ӻ���
��4��������NaBF4�������Σ���˺��еĵĻ�ѧ������Ϊ���Ӽ��ͼ��Լ������ڷ�ԭ�Ӻ��й¶Ե��ӣ����Ի�������λ����ͬ������ԭ��Bԭ��û�й¶Ե��ӣ�������ṹ�����������Σ�������109028���� Bԭ����sp3�ӻ���
��5������Bԭ����Nԭ��֮��������Ϊ���Լ����������֮���Ƿ��Ӽ�����������ΪBԭ�����γ�3��B��N�������Ժ�1 mol BN���������������к�B-N�ĸ���Ϊ3NA��
B2O3+3CaF2+3H2SO4
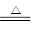 2BF3��+3CaSO4 +3H2O��B2O3+2NH3
2BF3��+3CaSO4 +3H2O��B2O3+2NH3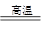 2BN+3H2O��
2BN+3H2O����2�����ݹ���ԭ����д����̬Bԭ�ӵĵ����Ų�ͼ����Ԫ���ǵڢ�A����ͼ��ǣ�3�ۣ�����BԪ�صĻ��ϼ��ǣ�3�ۡ�
��3����BF3�����У�����ԭ��Bԭ��û�й¶Ե��ӣ�������ṹ��ƽ�������Σ�������120�㡣����Bԭ�ӵ��ӻ��������Ϊsp2�ӻ���
��4��������NaBF4�������Σ���˺��еĵĻ�ѧ������Ϊ���Ӽ��ͼ��Լ������ڷ�ԭ�Ӻ��й¶Ե��ӣ����Ի�������λ����ͬ������ԭ��Bԭ��û�й¶Ե��ӣ�������ṹ�����������Σ�������109028���� Bԭ����sp3�ӻ���
��5������Bԭ����Nԭ��֮��������Ϊ���Լ����������֮���Ƿ��Ӽ�����������ΪBԭ�����γ�3��B��N�������Ժ�1 mol BN���������������к�B-N�ĸ���Ϊ3NA��

��ϰ��ϵ�д�
�����Ŀ
 Mg2+�����е����Ӻ�����
Mg2+�����е����Ӻ�����