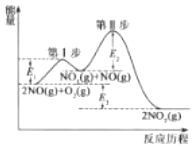
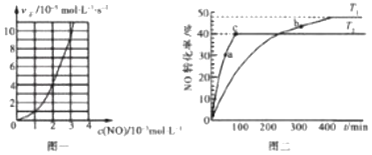
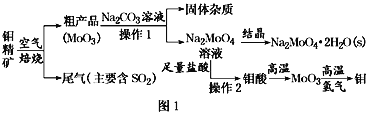
��Ŀ����
����Ŀ��O2��O3����Ԫ�ص����ֵ��ʣ����������ʽ������и��⣺
(1)���µ�ѹ�£���������O2��O3�������Ӹ�����Ϊ________��ԭ�Ӹ�����Ϊ________�����֮��Ϊ________��
(2)��NAΪ�����ӵ���������ֵ�����a g�����к��еķ�����Ϊb����c g�����ڱ�״���µ����Լ��________(�ú�NA��ʽ�ӱ�ʾ)��
(3)����500 mL 1 mol/L��ϡH2SO4��Һ����Ҫ����Ͳ��ȡŨH2SO4(�ܶ�Ϊ1.84 g/mL����������Ϊ98%)�����Ϊ________ mL��
���𰸡�3��2 1��1 3��2 ![]() L 27.2
L 27.2
��������
(1)����n=![]() ������������ʵ�����Ȼ�����N=n��NA���������Ŀ��n=
������������ʵ�����Ȼ�����N=n��NA���������Ŀ��n=![]() �������庬��ԭ�ӵ����ʵ������ٸ���V=n��Vm��������������
�������庬��ԭ�ӵ����ʵ������ٸ���V=n��Vm��������������
(2)�ȼ���c g O2���е������������Ȼ�����n=![]() ��V=n��Vm��������������
��V=n��Vm��������������
(3)����c=![]() �������Һ��Ũ�ȣ�Ȼ�����ϡ�Ͷ���cŨ��VŨ=cϡ��Vϡ�����㡣
�������Һ��Ũ�ȣ�Ȼ�����ϡ�Ͷ���cŨ��VŨ=cϡ��Vϡ�����㡣
(1)�������������Ϊm����n(O2)=![]() ��n(O3)=
��n(O3)=![]() �����Ե��µ�ѹ�£���������O2��O3�����ʵ����ı�Ϊn(O2)��n(O3)=
�����Ե��µ�ѹ�£���������O2��O3�����ʵ����ı�Ϊn(O2)��n(O3)=![]() ��
��![]() =3��2������N=n��NA��֪��������ʵ������京�е���������������ȣ�����N(O2)��N(O3)= n(O2)��n(O3)=3��2��
=3��2������N=n��NA��֪��������ʵ������京�е���������������ȣ�����N(O2)��N(O3)= n(O2)��n(O3)=3��2��
O2��O3������Oԭ�ӹ��ɣ�Oԭ�ӵ�������Ħ��������ͬ�������n=![]() ��֪���µ�ѹ�£���������O2��O3����ԭ�Ӹ�����ͬ����˺��е�ԭ�����ı�Ϊ1��1��
��֪���µ�ѹ�£���������O2��O3����ԭ�Ӹ�����ͬ����˺��е�ԭ�����ı�Ϊ1��1��
����V=n��Vm��֪������������������ʵ��������ȣ�����n(O2)��n(O3) =3��2������V(O2)��V(O3) =3��2��
(2)a g�����к��еķ�����Ϊb����c g O2���е����������N(O2)=![]() �������n=
�������n=![]() ��֪O2�����ʵ���Ϊn(O2)=
��֪O2�����ʵ���Ϊn(O2)=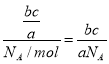 mol�����Ը������ڱ�״���µ����V=n��Vm=
mol�����Ը������ڱ�״���µ����V=n��Vm=![]() mol��22.4 L/mol=
mol��22.4 L/mol=![]() L��
L��
(3)�ܶ�Ϊ1.84 g/mL����������Ϊ98%��ŨH2SO4�����ʵ���Ũ��c=![]() mol/L=18.4 mol/L������ϡ��ʽ�ɵã�18.4 mol/L��V=500 mL��1 mol/L�����V=27.2 mL��
mol/L=18.4 mol/L������ϡ��ʽ�ɵã�18.4 mol/L��V=500 mL��1 mol/L�����V=27.2 mL��

����Ŀ�����Ȼ�����(S2Cl2)��һ����Ҫ�Ļ���ԭ��,����������,�ı��������ȷ�ճ�������Ӳ�����ʡ��������Ͽ�֪S2Cl2������������:
�������� | ���� | ɫ̬ | �ӷ��� | �۵� | �е� |
�綾 | ���ɫҺ�� | �ӷ� | -76�� | 138�� | |
��ѧ���� | ��300 ��������ȫ�ֽ� ��S2Cl2��Cl2 �������Ȼ�������Ӵ�,������ȼ�յ�Σ�� �����Ȼ���ˮ�ֽ����,�ų���ʴ������ | ||||
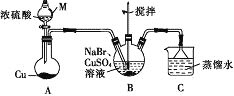
(1)��ȡ����S2Cl2
ʵ���ҿ�������������������110~140�淴Ӧ�Ƶ�S2Cl2��Ʒ�����������������SCl2��

������m ������Ϊ__________,װ��F ���Լ���������_________��
��װ������˳��: A![]() ______
______![]()
![]()
![]() E
E![]() D��
D��
��ʵ��ǰ��K1,ͨ��һ��ʱ��ĵ����ž�װ���ڿ�����ʵ�����ֹͣ���Ⱥ���ͨ��һ��ʱ��ĵ���,��Ŀ����_____________��
��Ϊ�����S2Cl2�Ĵ���,ʵ��Ĺؼ��ǿ��ƺ��¶Ⱥ�____________��
(2)����S2Cl2й©ʱӦ��ˮ��������ӷ�(����ɢ),��������������Һ,����Ҫ��й©���й©��ֱ����ˮ,��ԭ����______________��
(3)S2Cl2��ˮ������SO2��HCl��������,ijͬѧ���������ʵ�鷽�����ⶨ�û��������SO2�����������

��W��Һ������_____(����)��
a.H2O2��Һ b.KMnO4��Һ(�����ữ) c.��ˮ
�ڸû�������ж���������������Ϊ_________(�ú�V��m ��ʽ�ӱ�ʾ)��