��Ŀ����
15����֪��ϩ�ܷ�������ת����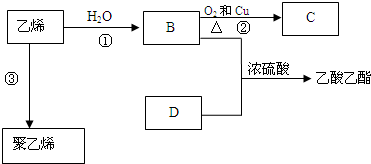
��1����ϩ�ĽṹʽΪ��
 ��
����2��д������������ŵĻ�ѧʽ�����ƣ�B�к������������ǻ���D�к������������Ȼ���
��3��д����Ӧ�Ļ�ѧ����ʽ
�٣�CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH ��Ӧ���ͣ��ӳɷ�Ӧ
�ڣ�2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O ��Ӧ���ͣ�������Ӧ
�ۣ�nCH2�TCH2$\stackrel{����}{��}$
 ��Ӧ���ͣ��Ӿ۷�Ӧ
��Ӧ���ͣ��Ӿ۷�Ӧ����������CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O��Ӧ���ͣ�������Ӧ��
���� ��ϩ�ķ���ʽΪC2H4������1��C=C�����������и�����ת����ϵ����ϩ��ˮ�ӳɵ�BΪCH3CH2OH���Ҵ���ͭ�������������·���������Ӧ��CΪCH3CHO���������Ҵ�����������Ӧ����������������DΪCH3COOH����ϩ�����Ӿ۷�Ӧ�þ���ϩ���ݴ˴��⣮
��� �⣺��ϩ�ķ���ʽΪC2H4������1��C=C�����������и�����ת����ϵ����ϩ��ˮ�ӳɵ�BΪCH3CH2OH���Ҵ���ͭ�������������·���������Ӧ��CΪCH3CHO���������Ҵ�����������Ӧ����������������DΪCH3COOH����ϩ�����Ӿ۷�Ӧ�þ���ϩ��
��1����ϩ�ķ���ʽΪC2H4������1��C=C�����ṹʽ ��
��
�ʴ�Ϊ�� ��
��
��2��BΪCH3CH2OH�������ǻ���DΪCH3COOH�������Ȼ���
�ʴ�Ϊ���ǻ����Ȼ���
��3����������ķ�����֪����Ӧ��Ϊ��ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ����÷�ӦΪCH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH��
��Ӧ��Ϊ�Ҵ�������������Ӧ������ȩ����BΪ�Ҵ���CΪ��ȩ���÷�ӦΪ2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��Ӧ��Ϊ��ϩ�ļӾ۷�Ӧ����Ӧ����ʽΪnCH2�TCH2$\stackrel{����}{��}$ ��
��
�����������ķ�Ӧ����ʽΪCH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O���÷�ӦΪ������Ӧ��
�ʴ�Ϊ��CH2=CH2+H2O$\stackrel{����}{��}$CH3CH2OH���ӳɷ�Ӧ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��������Ӧ��nCH2�TCH2$\stackrel{����}{��}$ ���Ӿ۷�Ӧ��CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O��������Ӧ��
���Ӿ۷�Ӧ��CH3COOH+CH3CH2OH$��_{��}^{Ũ����}$CH3COOCH2CH3+H2O��������Ӧ��
���� ���⿼���л����ƶϣ��漰ϩ�봼��ȩ������֮���ת�����ѶȲ���ע�����֪ʶ���������գ�

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�| A�� | ������������������ | |
| B�� | ��ϵ�д��������������ij����ܽ�ƽ�� | |
| C�� | ��Һ�в��ٴ���Fe3+ | |
| D�� | �����������ᣬ����Һ��Fe3+Ũ�Ȼ����� |
| A�� | ̼��ά��ǿ���ϲ��Ͽ�����������֯���ʹ������� | |
| B�� | �������մ����������ǽ������� | |
| C�� | ����ͭ����������ɫ�������� | |
| D�� | ̼�����մɾ���ѹ��ЧӦ |
| A�� | ��ˮ���ȵ�100�棬pH=6��c��H+����c��OH-�� | |
| B�� | �����£�pH=7�Ĵ���ʹ����ƵĻ����Һ�У�c��CH3COO-����c��Na+�� | |
| C�� | 0.1mol•L-1 ���������Һ�У�c��NH4+����c��SO42-����c��H+�� | |
| D�� | ͬŨ�ȵ�������Һ����CH3COONH4��NH4Cl��NH3•H2O�У�c��NH4+���ɴ�С��˳���Ǣڢۢ� |
 ����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺
����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺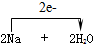 =2NaOH+H2����
=2NaOH+H2����