��Ŀ����
����Ŀ������ʵ������������( )
A. ����������ƽ��С�ձ���ȡ 10.2 g NaOH����
B. ijʵ����Ҫ 900 mL 0.1 mol/L������ͭ��Һ�������Ƹ���Һ���ȡ 22.5 g ��������
C. �� 10 mL ��Ͳ��ȡ 5.2 mL ���ᣬ����ʱʵ�����õ�Һ��������� 5.2 mL
D. ������һ�����ʵ���Ũ����Һ�Ķ��ݲ���ʱ�����Ӱ�Һ����������ҺŨ��ƫ��
���𰸡�B
���������������ƹ����ڿ����г��⣬���Գ���ʱҪʹ��С�ձ������IJ���������ѡ��A��ȷ��ʵ����û��900mL������ƿ������Ӧ������1000mL��0.1mol/L������ͭ��Һ����Ҫ0.1mol����������Ϊ25g��ѡ��B������ 10 mL ��Ͳ��ȡ 5.2 mL ���ᣬ���ӣ����Ͽ���ʱ��ʵ��Һ��ĸ߶Ȼ����5.2mL�Ŀ̶ȣ�����ʵ�����õ�Һ��������� 5.2 mL���ο���ͼ��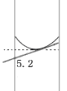 ��ѡ��C��ȷ��������һ�����ʵ���Ũ����Һ�Ķ��ݲ���ʱ�����ӣ����¿�����Һ�棬������Һ��������С��������ҺŨ��ƫ�ߣ��ο���ͼ��
��ѡ��C��ȷ��������һ�����ʵ���Ũ����Һ�Ķ��ݲ���ʱ�����ӣ����¿�����Һ�棬������Һ��������С��������ҺŨ��ƫ�ߣ��ο���ͼ��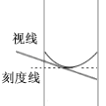 ��ѡ��D��ȷ��
��ѡ��D��ȷ��

����Ŀ���Ի�ͭ����Ҫ�ɷֶ�������ͭCuFeS2��Ϊԭ�ϣ���Fe2(SO4)3��Һ����ȡ����ȡͭ���ܷ�Ӧ�����ӷ���ʽ��CuFeS2 + 4Fe3+ ![]() Cu2+ + 5Fe2+ + 2S��
Cu2+ + 5Fe2+ + 2S��
��1���÷�Ӧ�У�Fe3+����________�ԡ�
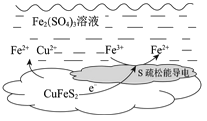
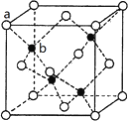
��2�������ܷ�Ӧ��ԭ����ͼ��ʾ��
�����ĵ缫��Ӧʽ��________��
��3��һ���¶��£����ƽ�ȡ��pH = 1��ȡ������ͬ������ͭ���ĩ�ֱ��������ʵ�飺
ʵ�� | ���� | 2Сʱ��Cu2+������/% |
I | ��������0.10 mol��L��1 Fe2(SO4)3��Һ | 78.2 |
II | ��������0.10 mol��L��1 Fe2(SO4)3��Һ��ͨ����� | 90.8 |
III | ��������0.10 mol��L��1 Fe2(SO4)3��Һ���ټ�������0.0005 mol��L��1 Ag2SO4��Һ | 98.0 |
�ٶԱ�ʵ��I��II��ͨ�������Cu2+��������ߵ�ԭ����________��
����ʵ��III�Ʋ⣬�ڽ�ȡCu2+������Ag+����������ԭ���ǣ�
����CuFeS2+4Ag+==Fe2++Cu2++2Ag2S
����Ag2S+2Fe3+==2Ag++2Fe2++S
Ϊ֤���ô�ԭ������������ʵ�飺
a��ȡ������ͭ���ĩ����������0.0005 mol��L��1 Ag2SO4��Һ����ֻ�Ϻ��á�ȡ�ϲ���Һ������ϡ���ᣬ�۲쵽��Һ��________��֤��������Ӧi��
b��ȡ����Ag2S��ĩ������________��Һ����ֻ�Ϻ��á�ȡ�ϲ���Һ������ϡ���ᣬ�а�ɫ������֤��������Ӧii��
��4����ʵ��II�Ľ�ȡҺ�����ȡͭ��ԭ����ͼ��ʾ��
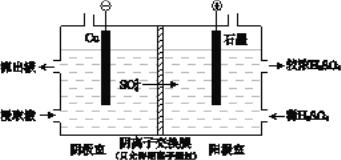
�� �����ڣ�����û��ͭ�������õ缫��Ӧʽ����ԭ����_______________��
�� �������ҵ�����Һ���������ң���ʹ��ȡ��������������ԭ���� _____________________��
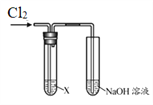
����Ŀ��ijѧϰС������ͼװ���о�Cl2�����ʡ�
| ��� | X | ʵ������ |
�� | AgNO3��Һ | ����a | |
�� | ���۵⻯����Һ | ��Һ���� | |
�� | ��ɫʯ����Һ | ��Һ�ȱ�����ɫ | |
�� | Na2SO3��Һ | ���������� |
��ش�
��1��ʵ����У�����a��______��
��2������ʵ����ƶ�Cl2�Ļ�ѧ������______��
��3������ʵ���ϻ�ѧ����ʽ˵�����������ԭ��______��
��4��ʵ��������������֤����Ӧ�����ˣ�����ʵ�鷽��______��
��5��������ӷ���ʽ˵��ʵ����NaOH��Һ��������______��