题目内容
 原子序数小于36的X、Y、Z、W四种元素,其中X原子基态时2p原子轨道上有3个未成对电子,Y原子基态时最外层电子数是其内层电子数的3倍,Z元素的最高价氧化物的水化物的酸性最强,W的原子序数为30.
原子序数小于36的X、Y、Z、W四种元素,其中X原子基态时2p原子轨道上有3个未成对电子,Y原子基态时最外层电子数是其内层电子数的3倍,Z元素的最高价氧化物的水化物的酸性最强,W的原子序数为30.(1)元素W的电子排布式为
(2)X、Y与氢元素形成的化合物XH2-YH中采用sp3杂化的原子有
(3)ZO4-离子的空间构型为
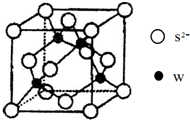
(4)元素W的一种硫化物晶体的晶胞结构如右上图所示,该硫化物的化学式是
分析:原子序数小于36的X、Y、Z、W四种元素,其中X原子基态时2p原子轨道上有3个未成对电子,则X原子核外电子排布为1s22s22p6,则X为N元素;Y原子基态时最外层电子数是其内层电子数的3倍,最外层电子数不超过8,所以内层电子数只能是2,最外层电子数为6,则Y是O元素;Z元素的最高价氧化物的水化物的酸性最强,则Z为Cl元素;W的原子序数为30,则W为Zn元素,据此解答.
解答:解:原子序数小于36的X、Y、Z、W四种元素,其中X原子基态时2p原子轨道上有3个未成对电子,则X原子核外电子排布为1s22s22p6,则X为N元素;Y原子基态时最外层电子数是其内层电子数的3倍,最外层电子数不超过8,所以内层电子数只能是2,最外层电子数为6,则Y是O元素;Z元素的最高价氧化物的水化物的酸性最强,则Z为Cl元素;W的原子序数为30,则W为Zn元素,
(1)元素W的核外电子数为30,其核外电子排布式为1s22s22p63s23p63d104s2,
故答案为:1s22s22p63s23p63d104s2;
(2)N、O与氢元素形成的化合物NH2-OH中N、O原子价层电子数为都是4,故N、O原子采用sp3杂化,1个NH2-OH分子中含有4个单键,故1molNH2-OH中含有σ键的数目为4NA,
故答案为:N、O;4NA;
(3)ClO4-离子中Cl原子价层电子对数=4+
=4,Cl原子没有孤对电子,故空间构型为正四面体型,与它互为等电子体的离子有SO42-、PO43-等,
故答案为:正四面体型;SO42-、PO43-;
(4)元素Zn的一种硫化物晶体,由晶胞结构可知,晶胞中Zn原子数目为4,S原子数目为8×
+6×
=4,故该硫化物的化学式是ZnS.元素Zn的氢氧化物可溶于氨水中,生成和铜氨配离子相同配位数的离子,生成配离子为[Zn(NH3)4]2+,则该反应的离子方程式为:Zn(OH)2+4NH3?H2O=[Zn(NH3)4]2++2OH-+4H2O,
故答案为:ZnS;Zn(OH)2+4NH3?H2O=[Zn(NH3)4]2++2OH-+4H2O.
(1)元素W的核外电子数为30,其核外电子排布式为1s22s22p63s23p63d104s2,
故答案为:1s22s22p63s23p63d104s2;
(2)N、O与氢元素形成的化合物NH2-OH中N、O原子价层电子数为都是4,故N、O原子采用sp3杂化,1个NH2-OH分子中含有4个单键,故1molNH2-OH中含有σ键的数目为4NA,
故答案为:N、O;4NA;
(3)ClO4-离子中Cl原子价层电子对数=4+
| 7+1-2×4 |
| 2 |
故答案为:正四面体型;SO42-、PO43-;
(4)元素Zn的一种硫化物晶体,由晶胞结构可知,晶胞中Zn原子数目为4,S原子数目为8×
| 1 |
| 8 |
| 1 |
| 2 |
故答案为:ZnS;Zn(OH)2+4NH3?H2O=[Zn(NH3)4]2++2OH-+4H2O.
点评:本题以元素推断为载体,综合考查物质结构与性质,涉及核外电子排布规律、杂化理论与价层电子对互斥理论、化学键、分子构型、晶胞计算、配合物等,难度中等,(4)注意利用均摊法进行晶胞计算,需要学生具备扎实的基础与综合运用能力.

练习册系列答案
相关题目
 (2011?江苏)原子序数小于36的X、Y、Z、W四种元素,其中X是形成化合物种最多的元素,Y原子基态时最外层电子数是其内层电子数的2倍,Z原子基态时2p原子轨道上有3个未成对的电子,W的原子序数为29.
(2011?江苏)原子序数小于36的X、Y、Z、W四种元素,其中X是形成化合物种最多的元素,Y原子基态时最外层电子数是其内层电子数的2倍,Z原子基态时2p原子轨道上有3个未成对的电子,W的原子序数为29. 原子序数小于36的x、Y、z、w四种元素,元素x的原子核外最外层电子数是内层电子数的2倍,元素Y与x同周期,其基态原子占据s轨道的电子数与占据p轨道的电子数相同,z是x的同族相邻元素;w是第Ⅷ族元素中原子序数最小的元素.用元素符号回答下列问题:
原子序数小于36的x、Y、z、w四种元素,元素x的原子核外最外层电子数是内层电子数的2倍,元素Y与x同周期,其基态原子占据s轨道的电子数与占据p轨道的电子数相同,z是x的同族相邻元素;w是第Ⅷ族元素中原子序数最小的元素.用元素符号回答下列问题:
