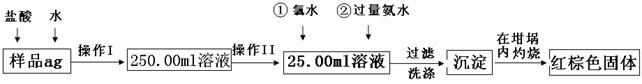
��Ŀ����
CuI��һ�ֲ�����ˮ�İ�ɫ���壬�������ɷ�Ӧ��2Cu2++4I-=2CuI��+I2���õ�������ʯīΪ��������CuΪ�������KI��Һ��ͨ��ǰ����Һ�м���������̪�͵�����Һ����ʼ���ã���������Һ�ʺ�ɫ������������Һ����ɫ��ͬʱ�а�ɫ�������ɡ�����˵������ȷ����
| A������������Һ����ɫ����ȷ�����ǣ�2I--2e-=I2���������۱��� |
| B������������Һ����ɫ����ȷ�����ǣ�Cu-2e-=Cu2+��Cu2+����ɫ |
| C����������Һ�ʺ�ɫ��ԭ���ǣ�2H++2e-=H2����ʹ��������OH-Ũ��������Һ�Լ��ԣ��Ӷ�ʹ��̪��� |
| D�������ϵĵ缫��ӦʽΪ��Cu2++2e-=Cu |
C
ͭ������������������ͭʧȥ���ӣ�A����ȷ������ͭ���������������ӣ����ɵ��ʵ�������۱�����B����ȷ�������ǵõ����ӵģ�����ѡ��C��ȷ��D����ȷ����ѡC��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 �����ڿ������ױ���������ȡʱ���ѹ۲쵽��ɫ��������ֻ�ܿ�������ɫ������ͼװ��ʹ��
�����ڿ������ױ���������ȡʱ���ѹ۲쵽��ɫ��������ֻ�ܿ�������ɫ������ͼװ��ʹ��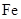 ��
�� ��ϡ����NaOH��Һ���ڻ�ԭ����������ȡ
��ϡ����NaOH��Һ���ڻ�ԭ����������ȡ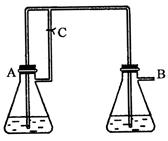
 mL bmol��L
mL bmol��L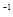 ��������Һ�У�����ʹ���ַ�Ӧ������NO��Ψһ�Ļ�ԭ����)������˵����ȷ���ǣ�������
��������Һ�У�����ʹ���ַ�Ӧ������NO��Ψһ�Ļ�ԭ����)������˵����ȷ���ǣ�������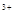
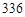 mL���壨��״��������b
mL���壨��״��������b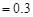
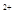 ʱ����a��b�Ĺ�ϵΪ��b��
ʱ����a��b�Ĺ�ϵΪ��b��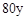 (1
(1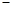 a/3)
a/3)