��Ŀ����
4����1����ҵ�ϣ���CO2��NH3Ϊԭ����һ�������ºϳ����صĻ�ѧ����ʽΪ��2NH3��g��+CO2��g��?CO��NH2��2��s��+H2O��g��
д���÷�Ӧƽ�ⳣ���ı���ʽ$\frac{c��{H}_{2}O��}{{c}^{2}��N{H}_{3}��c��C{O}_{2}��}$��������������ʱ����ѹǿ����������ƽ����Է���������ȷ�������������С���������䡱����ȷ��������
��2���ϳɰ���ԭ��֮һΪ����������Ȼ��Ϊȼ�Ϻϳ�������ԭ�����£�
��CH4��g��+H2O��g��?CO��g��+3H2��g����H=+206.4 kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.2 kJ•mol-1
�������ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽΪCH4��g��+2H2O��g��?CO2��g��+4H2��g����H=+165.2kJ•mol-1��
��3�����ڣ�2���еķ�Ӧ�٣����д�ʩһ����ʹƽ����ϵ�е�H2��������������AD
A�������¶�
B������ˮ����Ũ��
C�����������
D����Сѹǿ��
���� ��1����ѧ��Ӧ��ƽ�ⳣ��K=$\frac{������ƽ��Ũ���ݴη��˻�}{��Ӧ��ƽ��Ũ���ݴη��˻�}$��������������ʱ����ѹǿ��ƽ�������������С�ķ�����У��÷�Ӧ������������еķ�Ӧ��������Ҳ��С����M=$\frac{m}{n}$��ֵ��ȷ����
��2�������Ȼ�ѧ����ʽ��˹���ɼ���õ������Ȼ�ѧ����ʽ����+�ڵõ���
��3����CH4��g��+H2O��g��?CO��g��+3H2��g����H=+206.4 kJ•mol-1
��Ӧ�����������������ȷ�Ӧ��һ����ʹƽ����ϵ�е�H2�����������ƽ��������У��Ҳ������������������������ƽ���ƶ�ԭ�������ж�ѡ�
��� �⣺��1��2NH3��g��+CO2��g��?CO��NH2��2��s��+H2O��g��
��ѧ��Ӧ��ƽ�ⳣ��K=$\frac{������ƽ��Ũ���ݴη��˻�}{��Ӧ��ƽ��Ũ���ݴη��˻�}$=$\frac{c��{H}_{2}O��}{{c}^{2}��N{H}_{3}��c��C{O}_{2}��}$��������������ʱ����ѹǿ��ƽ�������������С�ķ�����У��÷�Ӧ������������еķ�Ӧ��������Ҳ��С����M=$\frac{m}{n}$��ֵ��ȷ����
�ʴ�Ϊ��$\frac{c��{H}_{2}O��}{{c}^{2}��N{H}_{3}��c��C{O}_{2}��}$����ȷ����
��2����CH4��g��+H2O��g��?CO��g��+3H2��g����H=+206.4 kJ•mol-1
��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.2 kJ•mol-1
�������ˮ������Ӧ���ɶ�����̼���������Ȼ�ѧ����ʽ�����Ȼ�ѧ����ʽ��˹���ɼ����+�ڵõ������Ȼ�ѧ����ʽ��CH4��g��+2H2O��g��?CO2��g��+4H2��g����H=+165.2kJ•mol-1��
�ʴ�Ϊ��CH4��g��+2H2O��g��?CO2��g��+4H2��g����H=+165.2kJ•mol-1��
��3����CH4��g��+H2O��g��?CO��g��+3H2��g����H=+206.4 kJ•mol-1
��Ӧ�����������������ȷ�Ӧ��һ����ʹƽ����ϵ�е�H2�����������ƽ��������У��Ҳ������������������������ƽ���ƶ�ԭ�������ж�ѡ�
A����Ӧ�����ȷ�Ӧ�������¶�ƽ��������У�H2�����������A��ȷ��
B������ˮ����Ũ�ȣ��������ת���ʣ������������������һ������B����
C�������Ч�����ı䷴Ӧ���ʣ����ı仯ѧƽ�⣬�����������䣬��C����
D����Сѹǿ��ƽ��������У����������������D��ȷ��
��ѡAD��
���� ���⿼�����Ȼ�ѧ����ʽ��д��������ѧƽ��Ӱ�����ط�����ƽ�ⳣ������ļ���Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ԭ�Ӱ뾶��Na��Mg��Al | B�� | ���ȶ��ԣ�HCl��H2S��PH3 | ||
| C�� | ����ǿ����H2SiO3��H2CO3��HNO3 | D�� | ����ǿ����KOH��NaOH��LiOH |
| A�� | 56g N2��12g H2���ܱ������з�Ӧ��ת�Ƶ��ӵ���ĿΪ12.0��6.02��1023�� | |
| B�� | ��״���£�22.4L NO��11.2L O2��Ϻ�����ķ�������Ϊ6.02��1023 | |
| C�� | ��ҵ���õ�ⷨ���д�ͭ����ʱ��ÿת��1mol���ӣ��������ܽ��ͭԭ�ӱ�Ϊ0.5��6.02��1023 | |
| D�� | V L a mol•L-1 �Ȼ�����Һ�У���Fe3+����ĿΪ6.02��1023����Cl-����Ŀ����3��6.02��1023 |
| ʵ�� | �����Լ� | ʵ��Ŀ�� | |
| �� | ��ʯ��ˮ��Ӧ | CuSO4��Һ | ��KMnO4������Һ������Ȳ�Ļ�ԭ�� |
| �� | CH3CH2Br��NaOH��Һ���� | HNO3��Һ | ��AgNO3��Һ����CH3CH2Br�е�Br |
| �� | ������ϡH2SO4ˮԡ���� | NaOH��Һ | ��������Һ����ˮ�����Ļ�ԭ�� |
| �� | C2H5OH��ŨH2SO4������170�� | NaOH��Һ | ��Br2��CCl4��Һ֤���÷�ӦΪ��ȥ��Ӧ |
| A�� | �٢ڢۢ� | B�� | ֻ�Т٢ڢ� | C�� | ֻ�Тڢۢ� | D�� | ֻ�Т٢ڢ� |
| A�� | ���� | B�� | �� | C�� | �ط� | D�� | ���Ϸ� |
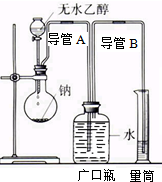 Ϊ��ȷ���Ҵ����ӵĽṹ��ʽCH3-O-CH3����CH3CH2OH��ʵ����������ͼ��ʾ��ʵ��װ�ã��ⶨ�Ҵ����Ʒ�Ӧ����H��0��������������������ݴ˼����Ҵ���������������Ʒ�Ӧ����ԭ�ӵ���Ŀ���Իش��������⣺
Ϊ��ȷ���Ҵ����ӵĽṹ��ʽCH3-O-CH3����CH3CH2OH��ʵ����������ͼ��ʾ��ʵ��װ�ã��ⶨ�Ҵ����Ʒ�Ӧ����H��0��������������������ݴ˼����Ҵ���������������Ʒ�Ӧ����ԭ�ӵ���Ŀ���Իش��������⣺