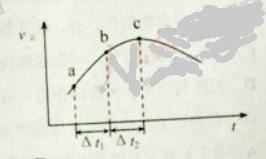
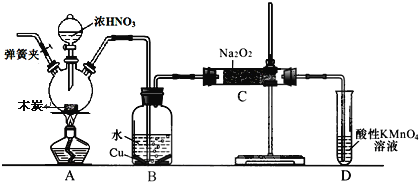
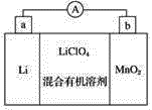
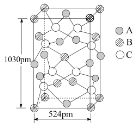
��Ŀ����
����Ŀ�������й���Һ������Ũ�ȵ�˵������ȷ����
A.0.1mol��L-1NaHCO3��Һ��c(Na+)>c(CO32-)>c(H2CO3)>c(OH-)>c(H+)
B.Na2Sϡ��Һ��c(H+)=c(OH-)-2c(H2S)-c(HS-)
C.pH=1��NaHSO4��Һ��c(H+)=c(SO42-)+c(OH-)
D.�������ʵ�����NaHC2O4��Na2C2O4����Һ��2c(Na+)=3[c(HC2O4-)+c(C2O42-)+c(H2C2O4)]
���𰸡�A
��������
A. NaHCO3�������Na+��HCO3-��HCO3-�ᷢ���������ò���H+��CO32-��Ҳ�ᷢ��ˮ�����ò���H2CO3��OH-������c(Na+)>c(CO32-)������HCO3-�ĵ�������С����ˮ�����ã�����c(H2CO3)>c(CO32-)����Һ�г�HCO3-ˮ�����OH-������ˮ���������OH-������c(OH-)>c(H2CO3)��ˮ�������ü���������c(H2CO3)>c(H+)���ø���Һ����Ũ�ȹ�ϵΪ��c(Na+)>c(HCO3-)>c(OH-)>c(H2CO3) >c(CO32-)>c(H+)��A����
B.��Na2Sϡ��Һ�����������غ�ɵ�c(OH-)=c(H+)+c(HS-)+2c(H2S)������c(H+)=c(OH-)-2c(H2S)-c(HS-)��B��ȷ��
C.NaHSO4�ᷢ�����룺NaHSO4=Na++H++SO42-��������ˮ�ĵ���ƽ�⣺H2O![]() H++OH-�����Ը��������غ�͵���غ��֪pH=1��NaHSO4��Һ������Ũ��Ϊc(H+)=c(SO42-)+c(OH-)��C��ȷ��
H++OH-�����Ը��������غ�͵���غ��֪pH=1��NaHSO4��Һ������Ũ��Ϊc(H+)=c(SO42-)+c(OH-)��C��ȷ��
D.�������ʵ�����NaHC2O4��Na2C2O4����Һ�У����������غ�ɵã�2c(Na+)=3[c(HC2O4-)+c(C2O42-)+c(H2C2O4)]��D��ȷ��
�ʺ���ѡ����A��

 ����5��2���ϵ�д�
����5��2���ϵ�д�