��Ŀ����
6��������BN����һ����Ҫ�Ĺ����մɲ��ϣ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������BF3��BN����ش��������⣺��1����̬Bԭ�ӵĵ����Ų�ʽΪ1s22s2sp1�� BN��BԪ�صĻ��ϼ�ΪN��
��2����BF3�����У�F-B-F�ļ�����120�㣬Bԭ�ӵ��ӻ��������Ϊsp2��BF3����NaF���ÿ�����NaBF4��BF${\;}_{4}^{-}$�����幹��Ϊ�������壻
��3������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ���ۼ������Թ��ۼ����������������Ϊ���Ӽ���������
��4�������������ڸ��¸�ѹ�£�����ת��Ϊ���������������������е�ԭ������ԭ�ӽ������У��侧��ṹ����ʯ���ƣ�Ӳ������ʯ�൱�������߳�Ϊ3.615��10-10cm������������ľ���������ԭ�Ӿ��壬�����������о�������4����ԭ�ӣ��������ܶ���=$\frac{25��4}{��3.615��1{0}^{-10}��^{3}��{N}_{A}}$g•cm-3����ֻ����ʽ�����ؼ������ֵ������٤������ΪNA�����ԭ��������N-14 B-11 ����
���� ��1��Bԭ�Ӻ��������Ϊ5�������������ԭ����д��������Ų���B���ڵڢ�A��Ԫ�أ����ϼ�Ϊ+3�ۣ�
��2������Bԭ�Ӽ۲���Ӷ������µ��Ӷ�����ȷ��BF3���ӡ�BF4-�Ŀռ�ṹ���ж�BF3�м��ǡ�Bԭ���ӻ���ʽ��
��3��B��N�����ڷǽ���Ԫ�أ������γɵĻ�ѧ���Ǽ��Թ��ۼ���������ṹ��ʯī���ƣ������֮��Ӧ�ÿ����Ӽ���������ϣ�
��4�������������е�ԭ������ԭ�ӽ������У��侧��ṹ����ʯ���ƣ�Ӳ������ʯ�൱����������������ԭ�Ӿ��壻���ʯ�����������壬����8��������8��̼ԭ�ӣ�6�������6��̼ԭ�ӣ��������ڲ�����4��̼ԭ�ӣ���ͼ��ʾ��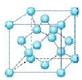 �����Խ��ʯ��һ�������к��е�̼ԭ����=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8�����������������Ӧ�ú���4��N��4��Bԭ�ӣ�����BN��������������������������ݦ�=$\frac{m}{V}$�����ܶȣ�
�����Խ��ʯ��һ�������к��е�̼ԭ����=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8�����������������Ӧ�ú���4��N��4��Bԭ�ӣ�����BN��������������������������ݦ�=$\frac{m}{V}$�����ܶȣ�
��� �⣺��1����̬Bԭ�ӵĵ����Ų�ʽΪ1s22s2sp1��BN��BԪ�صĻ��ϼ�Ϊ+3���ʴ�Ϊ��1s22s2sp1��+3��
��2��BF3��Bԭ�ӹ¶Ե��Ӷ���=$\frac{1}{2}$����3-3��1��=0��Bԭ���γ�3���� ��������BF3����Ϊƽ���������Σ�����Ϊ120�㣬�ӻ������ĿΪ3+0=3��Bԭ���ӻ���ʽΪsp2��BF4-����ԭ�ӵŶԵ��Ӷ���=$\frac{1}{2}$����3+1-4��1��=0��Bԭ���γ�4���� ����������ṹΪ�������壬�ʴ�Ϊ��120�㣻sp2���������壻
��3��B��N�����ڷǽ���Ԫ�أ������γɵĻ�ѧ���Ǽ��Թ��ۼ�������ʯī�ṹ��֪�������������У������֮�俿���Ӽ���������ϣ��ʴ�Ϊ�����ۼ������Թ��ۼ��������Ӽ���������
��4�������������е�ԭ������ԭ�ӽ������У��侧��ṹ����ʯ���ƣ�Ӳ������ʯ�൱����������������ԭ�Ӿ��壻���ʯ�����������壬����8��������8��̼ԭ�ӣ�6�������6��̼ԭ�ӣ��������ڲ�����4��̼ԭ�ӣ���ͼ��ʾ�� �����Խ��ʯ��һ�������к��е�̼ԭ����=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8�����������������Ӧ�ú���4��N��4��Bԭ�ӣ�BN�����е�����Ϊ$\frac{25g}{{N}_{A}}$��һ������������������ǣ�3.615��10-10cm��3�����������������ܶ�=$\frac{25g}{{N}_{A}}$�£�3.615��10-10cm��3=$\frac{25��4}{��3.615��1{0}^{-10}��^{3}��{N}_{A}}$g•cm-3��
�����Խ��ʯ��һ�������к��е�̼ԭ����=8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8�����������������Ӧ�ú���4��N��4��Bԭ�ӣ�BN�����е�����Ϊ$\frac{25g}{{N}_{A}}$��һ������������������ǣ�3.615��10-10cm��3�����������������ܶ�=$\frac{25g}{{N}_{A}}$�£�3.615��10-10cm��3=$\frac{25��4}{��3.615��1{0}^{-10}��^{3}��{N}_{A}}$g•cm-3��
�ʴ�Ϊ��ԭ�Ӿ��壻4��$\frac{25��4}{��3.615��1{0}^{-10}��^{3}��{N}_{A}}$��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų������ӿռ乹�͡��ӻ����͵��жϡ������ṹ�Լ��������йؼ��㣬��4��Ϊ�״��㣬ע�������ѧ���������ṹ���Ѷ��еȣ�

| A�� | �����CuO��NO��SO2��H2O | |
| B�� | �NaOH��KOH��Ba��OH��2��Na2CO3 | |
| C�� | ���������Na2O��CaO��Al2O3��Na2O2 | |
| D�� | ����ʣ�KNO3��Cl2��HCl��BaSO4 |
| A�� | һ���¶Ⱥ�ѹǿ�£���̬���ʵ������Ҫ�ɹ�������ķ��ӵĴ�С���� | |
| B�� | ��1 L 2 mol/L��H2SO4��Һ��ȡ��0.5 L������Һ�������ӵ�Ũ��Ϊ4mol/L | |
| C�� | ͬ��ͬѹ�£�30mLA2�����10mL B2����ǡ����ȫ��Ӧ����20mLC���壬��C��ѧʽΪ A3 B��B A3 | |
| D�� | ͬ��ͬѹ���κ�����ķ��Ӽ���뼸����� |
| A�� | ��ͬ������κ����嶼������ͬ��Ŀ�ķ��� | |
| B�� | ��״���£�5L H2S������5L NH3��ԭ�Ӹ�����Ϊ3��4�� | |
| C�� | ͬ��ͬѹ����ͬ�����N2����CO��������ͬ���ʵ�������ͬ����������ͬ�ĶԿ������ܶ� | |
| D�� | ��ͬ�¶��£������ѹǿ�ȵ�����������ķ��� |
| A�� | CS2ΪV�εļ��Է��� | |
| B�� | ClO3- �Ŀռ乹��Ϊƽ�������� | |
| C�� | SF6��Sԭ�Ӻͷ�ԭ�Ӿ����������8�����ȶ��ṹ | |
| D�� | SiF4��SO32-������ԭ�Ӿ�Ϊsp3�ӻ���SiF4���ӳʿռ��������壬SO32-�������� |
| A�� | ����������Һ�����ᷴӦ Ba2++OH-+H++SO42-=BaSO4��+H2O | |
| B�� | �����ʯ��ˮ��ϡ���ᷴӦ Ca��OH��2+2H+=Ca2++2H2O | |
| C�� | ͭƬ������������Һ�� Cu+Ag+=Cu2++Ag | |
| D�� | ̼�ᱵ����ϡ������ BaCO3+2H+=Ba2++H2O+CO2�� |
| A�� | H2O������ʱ��Cu��H2O�γɦҼ� | B�� | H2O����λ��������NH3 | ||
| C�� | �Ҵ��ɼ�С���Ӿ�����ܽ�� | D�� | Cu��NH3��4SO4��BaCl2�а�ɫ�������� |
| ���ӷ��� | ��Է��ӣ�ԭ�ӣ����� | ÿ�����ӵ�������g/���� | 1mol���ʺ��е������������� | 1mol����������g�� |
| C | 1.993��10-23 | |||
| Fe | 9.302��10-23 | |||
| H2SO4 | 1.628��10-22 | |||
| H2O | 2.990��10-23 | |||
| Na+ | 3.821��10-23 | |||
| OH- | 2.824��10-23 |
��1mol�κ�ԭ�ӵ���������ֵ�϶��������ԭ��������
��1mol�κη��ӵ���������ֵ�϶��������ַ��ӵ���Է���������
��1mol�κ����ӵ���������ֵ�϶������������ӵ����ԭ��������
| A�� | Al3+ H+ HCO3- | B�� | Na+ NO3- Cl- | C�� | H+ OH- SO42- | D�� | Ca2+ K+ CO32- |