��Ŀ����
16������˵��������ǣ�������| A�� | ��������ʵ�����ģ������������ޣ����ձ�������������ͷ�ιܡ���Һ©�������������ˮ��CCl4��ȥNaBr��Һ�е�����NaI��ʵ�� | |
| B�� | �Ʊ�������������ʱ�������������������Ũ����Һʱ��ֻ��С���������Һ��ȫ������ | |
| C�� | ����֧ʢ��KI3��Һ���Թ��зֱ������ۺ���������Һ��ǰ����Һ�������߲�����ɫ�������ɴ˵ó�KI3��Һ�д���ƽ��I3-?I2+I- | |
| D�� | ��ѧ�����о������۽ṹ�Ĺ������Ⱥ�ʹ���˹�ѧ����������������ɨ�����������ȹ۲����� |
���� A���õ���ȡ�ͷ�Һ�������õ��ձ�������������ͷ�ιܡ���Һ©����
B��������������ֱ�����ɣ�
C���������۱�����I-��AgNO3��Һ��Ӧ���ɻ�ɫ������
D����ѧ��������ֱ�ӹ۲�ԭ�Ӻͷ���ʵ��̫ңԶ�ˣ�������������Ȼ�ȹ�ѧ����ǰ����һ�������뿪ֱ�ӹ۲�ԭ�Ӻͷ��ӻ���һ�ξ��룮20����80�������չ������ɨ������������ʹ�����ܹ�ֱ�ӹ۲���о������۽ṹ������������
��� �⣺A������ˮ��CCl4��ȥNaBr��Һ�е�����NaI����Ҫ�ȵμ���ˮ����ȥ�⻯�ƣ�Ȼ��ͨ����ȡ����Һ�������õ��������ձ�������������ͷ�ιܡ���Һ©������A��ȷ��
B��������������ֱ�����ɣ��Է�ֹ�¶ȹ��߶����·ֽ�����ʣ���B����
C���������֪��KI3��Һ�д��� I3-?I2+I-ƽ�⣬�ֱ���������Һ��AgNO3��Һƽ��������ƶ�����C��ȷ��
D����ѧ��������ֱ�ӹ۲�ԭ�Ӻͷ���ʵ��̫ңԶ�ˣ�������������Ȼ�ȹ�ѧ����ǰ����һ�������뿪ֱ�ӹ۲�ԭ�Ӻͷ��ӻ���һ�ξ��룮20����80�������չ������ɨ������������ʹ�����ܹ�ֱ�ӹ۲���о������۽ṹ��������������D��ȷ��
��ѡB��
���� ���⿼�黯ѧʵ�����������ʵ��ԭ����ע��ʵ�����֪ʶ���飬�Ѷ��еȣ���Ҫѧ����������ʵ��ԭ����

 ��У����ϵ�д�
��У����ϵ�д�| A�� | 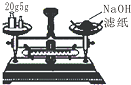 ����25g�������� ����25g�������� | B�� | 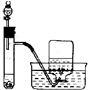 H2O2��Һ�Ʊ�O2 | C�� | 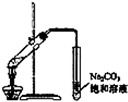 �����������Ʊ� �����������Ʊ� | D�� | 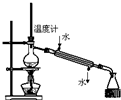 ʯ������ |
| A�� | Mg2+��Na+��Cl-��CO32- | B�� | Ba2+��K+��OH-��NO3- | ||
| C�� | H+��Al3+��NH4+��SO42- | D�� | Na+��Cl-��Ca2+��HCO3- |
 NH2+CHCOOH$\stackrel{��}{?}$
NH2+CHCOOH$\stackrel{��}{?}$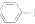
 +H2O
+H2O| ���� | ��Է������� | ��״ | �ܶ�/��g/cm3�� | �۵�/�� | �е�/�� | �ܽ�� | |
| ���� | 93 | ��ɫ��״Һ����л�ԭ�� | 1.02 | -6.1 | 184 | ����ˮ | ���������Ҵ������� |
| ���� | 60 | ��ɫҺ�� | 1.05 | 16.5 | 118 | ������ˮ | |
| ���� ���� | 135 | ��ɫ���� | 1.22 | 114 | 304 | ������ˮ��������ˮ | |
��50mlԲ����ƿ�м�����ˮ����5ml����������7.5mL��п��0��lg������ͼ��װ�����������ʯ������Ӧ�����ȼ��ȣ�ʹ��ӦҺ����״̬�»��������ڼ����¶ȣ�ʹ�����¶ȿ�����105�����ң���ӦԼ60��80nun������Ӧ�������ʱ��ֹͣ���ȣ�
�ڽ����£����Ƚ���ƿ�е����ϵ���ʢ��l00mL��ˮ���ձ��У����ҽ��裬����ȴ�ձ������£������������ᾧ���������ˡ�ϴ�ӡ�����õ�����������Ʒ������Ʒ�ؽᾧ�����ˣ����ɣ����أ�������ʣ�
ע��DΪ���η����������ڷе���̫��Ļ����ķ��룮
 ��
����ش��������⣺
��1������A�����������ܣ�
��2��װ��ͼ�м��ȿ�����ԡ���ˮԡ������ԡ������
��3��ʵ���м���п�۵�Ŀ���Ƿ�ֹ�����ڷ�Ӧ�����б�������
��4��Ϊ��Ҫ���Ʒ������϶˵��¶���105�����Ҳ��Ϸֳ���Ӧ���������ɵ�ˮ���ٽ���Ӧ������У����������IJ��ʣ�
��5��ͨ���۲쵽�¶ȼ��¶��½�����ƿ��Һ�岻�����ӣ�������жϷ�Ӧ������ɣ���Ӧ�����������������������õı�ˮ�е�ԭ�������������۵�ϸߣ����伴��̻�����������ƿ����������
��6��ϴ������������Ʒ����ʵ��Լ���a��
a����������ˮϴ b����������ˮϴc��������ˮϴ��������ˮϴ d���þƾ�ϴ
��7����ʵ�����յõ���Ʒ1.8g�������������IJ�����24%��
��֪����+3��Cr��������Һ�������ȶ�����pH��9ʱ��CrO42-��ʽ��������������
�ڳ����£�����������������������ʽ��������Һ��pH���£�
| �� | Fe | Mg | Al | Cr |
| ��ʼ����ʱ��H | 1.9 | 9.0 | -- | -- |
| ������ȫʱ��H | 3�� | 11.1 | 8 | 9������9�ܽ⣩ |
��2������II��Fe��OH��3��Al��OH��3������II�����ܷ�ʡ�ԣ�Ϊʲô��
���ܣ���pH=8ʱ��Al3+�Ѿ���ȫת��Ϊ���������������˳�ȥ������������NaOHʱ��Al��OH��3���ܽ⣬������������AlO2-��
��3��д����Һ�м���H2O2������Ӧ�����ַ���ʽ2Cr3++3H2O2+H2O=Cr2O72-+8H��
��4��ȡ�õ���Na2CrO4����0.48g������������Һ�õ�Na2CrO��Һ����0.3000 mol•L-1�ζ����յ�ʱ��������ԭΪCr3+��������20.00mL����Һ��
��ʢװFeSO4����ҺӦ����ʽ�ζ��ܣ����ʽ����ʽ�������ζ�ʱ�IJ���Ϊ�����ֿ��ƻ�������������ƿ���۾�ע����ƿ����ɫ�仯���ζ����յ�ʱ����¼��Һ�������
�ڼ���ò�Ʒ��Na2CrO4����������Ϊ66.67%��

| A�� | ��10 mL0.1 mol•L-1Na2CO3��Һ��εμӵ�10 mlL0.1 mol•L-1�����У������Һ�и�����Ũ�ȵĴ�С��ϵ��c��Na+����c��Cl-����c��HCO3-����c��CO32-�� | |
| B�� | ���ʵ���Ũ����ȵ�NaF��Һ��CH3COONa��Һ��Ƚϣ��������ӵ���Ũ����� | |
| C�� | ��0.1mol•L-1��FeCl3��Һ�еμ���������KMnO4��Һ��KMnO4��Һ����ɫ��˵��FeCl3ֻ�������ԣ��������������� | |
| D�� | ��AgCl��AgBr�ı�����Һ�������ϣ��ټ�������AgNO3Ũ��Һ���ɹ۲쵽��������ɫ������������ɫ������˵��Ksp��AgCl����Ksp��AgBr�� |
 ��ͭ��һ�㺬��п����������������ʣ�����ͼ��ʾ��װ���У��׳ص��ܷ�Ӧ����ʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O����ͨ��·һ��ʱ���Cu�缫����������3.2g���ڴ˹����У�����˵����ȷ���ǣ�������
��ͭ��һ�㺬��п����������������ʣ�����ͼ��ʾ��װ���У��׳ص��ܷ�Ӧ����ʽΪ��2CH3OH+3O2+4KOH�T2K2CO3+6H2O����ͨ��·һ��ʱ���Cu�缫����������3.2g���ڴ˹����У�����˵����ȷ���ǣ�������| A�� | �׳��е������Һ��pHֵ��С | |
| B�� | �ҳ���CuSO4��Һ��Ũ�Ȳ��� | |
| C�� | �׳������������Ŀ����������2.8L��������O2���������20%���㣩 | |
| D�� | �׳�ͨ��CH3OHһ���ĵ缫��ӦΪ��CH3OH-6e-+2H2O�TCO32-+8H+ |
| A�� | �ܢ٢ڢ� | B�� | �ܢۢڢ� | C�� | �ܢ٢ۢ� | D�� | �ڢۢ٢� |
| A�� | ������ˮϴ����ʽ�ζ��ܺ�װ���������еζ� | |
| B�� | ������ˮϴ����ƿ������NaOH��Һ��ϴ������װ��һ�������NaOH��Һ | |
| C�� | �ζ�����������Ӧһֱע����ƿ�ڻ��Һ��ɫ�ı仯 | |
| D�� | �÷�̪��ָʾ������Һ����ɫ��dz��ɫ������Ӳ���ɫΪ�ζ��յ� |