��Ŀ����
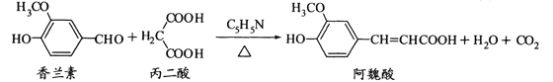
����Ŀ�����״��뱽��������Ҫ�Ļ���ԭ�ϣ���ͨ������ȩ����������ˮ��Һ�е��绯��Ӧ�Ƶã���ӦʽΪ��

ij�о�С����ʵ�����Ʊ����״��뱽���ᣬ��Ӧ������Է�ӦҺ�����в��账����

�ؽᾧ���̣��ܽ���������̿��ɫ�������ȹ���������ȴ�ᾧ������������ϴ����������
��֪�����״����������ѡ��Ҵ�����ˮ���ܽ�Ƚ�С������������ˮ��
�����������Ϣ���ش��������⣺
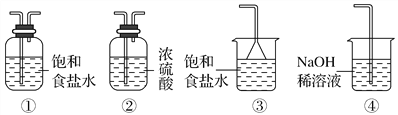
(1)��ȡ���뱽�״��뱽������ʱ�����ʵ���ȡ����_____________����������____________����ȡ��Һ������ˮ���������ữ��Ŀ����(���û�ѧ����ʽ��ʾ)_______________________________��
(2)��������A��B��C�����ܼ��е��ܽ��(s)���¶ȱ仯����������ͼ��ʾ��

�ؽᾧʱ�����ʵ��ܼ���_____________���ؽᾧ�����У����ȹ��˵�������________��ϴ��ʱ���õĺ���ϴ�Ӽ���_______��(������ĸ)
A������ʳ��ˮ B��Na2CO3��Һ C��ϡ���� D������ˮ
(3)Ϊ����ϳɲ����б�����ĺ�������ȡ����1.220 g���ܽ��������ƿ�ж�����100 mL����ȡ25.00 mL������Һ����0.1000 mol��L-1 NaOH��Һ�ζ����ζ����յ�ʱNaOH��Һ������24.65 mL���������б�����ĺ�����___________��
���𰸡����� ���״��������е��ܽ�ȴ�����ˮ�е��ܽ�ȣ���������ˮ�������� C6H5-COONa+HCl=C6H5-COOH��+NaCl C ��ȥ���������ʣ���ֹ��������ȴ��ᾧ���� D 98.60 %
��������
��ӦҺ���б��״��ͱ������ƣ�����������ȡ����Һ���õ����л���Ϊ���״������ѵĻ�������ɵõ����״���ˮ��Ϊ�������Ƶ�ˮ��Һ�����������ữ�õ������ᣬ�������ᾧ�����ˡ�ϴ�ӿɵõ������ᣬ�Դ˷������
(1) Ӧѡ�����ȡ�������ѣ�ԭ���DZ��״��������е��ܽ�ȴ�����ˮ�е��ܽ�ȣ���������ˮ�������ܣ�������������ɱ����ᣬ���ؽᾧ��������ѧ����ʽΪC6H5-COONa+HCl=C6H5-COOH��+NaCl��
��ˣ�������ȷ���ǣ����ѣ����״��������е��ܽ�ȴ�����ˮ�е��ܽ�ȣ���������ˮ�������ܣ�C6H5-COONa+HCl=C6H5-COOH��+NaCl��
(2)Ӧѡ���Լ�C��ԭ�������ܼ�C�����¶ȱ仯��������ܽ�ȱ仯�ϴ��������ؽᾧ���룬����ʱҪ���ȹ��ˣ��ɳ�ȥ���������ʣ���ֹ��������ȴ��ᾧ������ϴ��ʱ��������ˮ����ֹ�����������ʣ�ѡD��
��ˣ�������ȷ���ǣ�C����ȥ���������ʣ���ֹ��������ȴ��ᾧ������D��
(3)n��NaOH��=0.1000mol/L��24.65��10-3L=2.465��10-3mol��
�������б���������ʵ���Ϊ2.465��10-3mol��![]() =9.86��10-3mol��
=9.86��10-3mol��
����Ϊ9.86��10-3mol��122g/mol=1.203g��
�����б�����ĺ���Ϊ![]() ��100%=98.6%��
��100%=98.6%��
��ˣ�������ȷ���ǣ�98.60%��