��Ŀ����
����Ŀ��ij��ѧ����С���о���������������ʱ���ı�ijһ������3A2(g)+B2(g)2AB3(g)��ѧƽ��״̬��Ӱ�죬�õ���ͼ��ʾ�ı仯���ɣ�ͼ��T��ʾ�¶ȣ�n��ʾ���ʵ�������������ͼ�ɵó����жϽ�����ȷ��
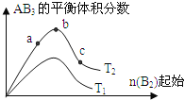
A. a��b��c����ƽ�ⳣ���Ĵ�С��ϵΪ��Kb��Kc ��Ka
B. �ﵽƽ��ʱB2��ת���ʴ�СΪ��b��a��c
C. ����ѹǿ������AB3�����ɣ������ʵ��������ѹǿԽ��Խ��
D. ����ʼ��Ϊ![]() ,����T2�¶��£�ƽ��ʱAB3����������ӽ�b��
,����T2�¶��£�ƽ��ʱAB3����������ӽ�b��
���𰸡�D
��������
A.��ͼ��֪��a��b��c�����������¶���ͬ������ƽ�ⳣ����С��ϵΪKb=Ka=Kc����A����B.���ڷ�Ӧ3A2(g)+B2(g)2AB3(g)����n(B2)�����ӷ�Ӧ������Ӧ�����ƶ�����n(B2)ת���ʽ��ͣ���B2��ת����ӦΪ��a��b��c����B����C. ����ѹǿƽ��������Ӧ�����ƶ�������AB3�����ɣ�����Ҫ�������������ͳɱ������Բ�����ѹǿԽ��Խ�ã���C����D.�ɷ�Ӧ3A2(g)+B2(g)2AB3��֪����![]() =
=![]() ʱAB3 ���������������ԣ���T2�¶��£�����ʼ��Ϊ
ʱAB3 ���������������ԣ���T2�¶��£�����ʼ��Ϊ![]() ,ƽ��ʱAB3����������ӽ�b�㣬��D��ȷ�����Ա����Ϊ��D��
,ƽ��ʱAB3����������ӽ�b�㣬��D��ȷ�����Ա����Ϊ��D��

����Ŀ����������ʵ��������������õ��Ľ��۲���ȷ����
ѡ�� | ʵ����������� | ʵ����� |
A | ����Һ�м���Na2CO3��Һ����Һ����� | ���ԣ�����>HCO |
B | ��pH�Ʋⶨ��Ũ�ȵ�Na2CO3��NaClO��Һ��pH | ����pH��ǰ�ߵĴ� |
C | �����Ҵ���Ӧƽ��������ˮ��Ӧ���� | �ǻ�����Ļ��ԣ�C2H5OH<H2O |
D | ��2 mL 0.01 mol��L��1��Na2S��Һ���ȵ��뼸��0.01 mol��L��1 ZnSO4��Һ�а�ɫ�������ɣ��ٵ���0.01 mol��L��1 CuSO4��Һ���ֳ��ֺ�ɫ���� | Ksp(CuS)<Ksp(ZnS) |
A. AB. BC. CD. D
����Ŀ�������������ڻ�ѧ��ҵ��������Ҫ��Ӧ�ã��ش��������⣺
(1)��N2O��NO��Ӧ����N2��NO2�������仯(��ʾ����1molN2�������仯)��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽΪ______________________��
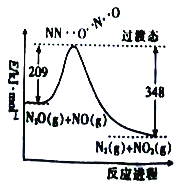
(2)һ�������£�����識��ȷֽ�õ��IJ���ֻ��N2O��H2O��250��ʱ����������ܱ������зֽ�ﵽƽ�⣬�÷ֽⷴӦ��ƽ�ⳣ������ʽΪK=___________������1mol�������ȫ�ֽ⣬��ת�Ƶ��ӵ���ĿΪ___________(��NAΪ�����ӵ�������ֵ)��
(3)���������������ڼ��������·�����Ӧ��O2NC6H4COOC2H5+OH��![]() O2NC6H4COO��+C2H5OH�����ַ�Ӧ��ij�ʼŨ�Ⱦ�Ϊ0.80mol��L��1��T��ʱ���O2NC6H4COOC2H5��ת��������ʱ��仯�����������ʾ��
O2NC6H4COO��+C2H5OH�����ַ�Ӧ��ij�ʼŨ�Ⱦ�Ϊ0.80mol��L��1��T��ʱ���O2NC6H4COOC2H5��ת��������ʱ��仯�����������ʾ��
t/s | 0 | 60 | 90 | 120 | 160 | 260 | 300 | 360 | 400 |
a/% | 0 | 33.0 | 41.8 | 48.8 | 58.0 | 69.0 | 70.4 | 71.0 | 71.0 |
�ٸ÷�Ӧ��60~90s��90~120s�ڵ�ƽ����Ӧ���ʷֱ�ԼΪ___________��___________���Ƚ����ߴ�С�ɵó��Ľ�����______________________��
�ڼ���T��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ______________________��
��Ϊ���O2NC6H4COOC2H5��ƽ��ת���ʣ������ʵ����Ʒ�Ӧ�¶��⣬�����Բ�ȡ�Ĵ�ʩΪ______________________(д��һ������)��