��Ŀ����
��֪H2A��ˮ�д�������ƽ�⣺H2A H����HA����HA��
H����HA����HA�� H����A2�����ش��������⣺
H����A2�����ش��������⣺
��1����֪�����£�H2A�ĸ���(CaA)������Һ�д���ƽ�⣺
CaA(s) Ca2��(aq)��A2��(aq)����H �� 0��
Ca2��(aq)��A2��(aq)����H �� 0��
���¶�����ʱ��Ksp________(���������С�����䡱��ͬ)��
�ڵμ�����Ũ���ᣬc (Ca2��)________��ԭ����____ ______________(�����ֺ����ӷ���ʽ˵��)��
��2������CaA����Һ�м���CuSO4��Һ������һ�ֺ�ɫ�������ʣ�д���ù����з�Ӧ�����ӷ���ʽ______________________����ijCuSO4��Һ��c (Cu2��)��0.02 mol/L�����Ҫ����Cu(OH)2������Ӧ������ҺpH��ʹ֮����________(��֪Ksp[Cu(OH)2]��2.0��10��20)��
��3�������£���ij������Һ�е����̪����Һ�ʺ�ɫ�������Һ��________�ԡ��ڷ�������Һ����̪�ʺ�ɫ��ԭ��ʱ����ͬѧ��Ϊ��������Һʱ���õĴ�����Ʒ�л���NaOH���£���ͬѧ��Ϊ����Һ�е������CO32-ˮ�����£��������һ����ʵ�鷽����������λͬѧ��˵����������(������Ҫ����������ͽ���)_________________ ___________��
��1������������ �����ᷢ����Ӧ��A2����H�� HA����A2��Ũ�ȼ�С��CaA���ܽ�ƽ�������ƶ���n(Ca2��)����������Һ����仯��������c(Ca2��)����
HA����A2��Ũ�ȼ�С��CaA���ܽ�ƽ�������ƶ���n(Ca2��)����������Һ����仯��������c(Ca2��)����
��2��CaA(s)��Cu2��(aq)=Ca2��(aq)��CuA(s) ��2�֣� 5��2�֣�
��3���� �� ����Ba2����ȥ̼������ӵĸ��ţ������Һ���Ժ�ɫ����֤����ͬѧ�Ĺ۵���ȷ���������̼���ˮ���Ե�ʣ�����ͬѧ�Ĺ۵���ȷ��������������Ҳ���֣�
���������������1����֪�����£�H2A�ĸ���(CaA)������Һ�д���ƽ�⣺CaA(s)? ?Ca2��(aq)��A2��(aq)����H �� 0���ٸ÷�ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���Ksp���ڸ��ݻ�ѧƽ���ƶ�ԭ�����μ�����Ũ���ᣬ������Ӧ��A2����H��
?Ca2��(aq)��A2��(aq)����H �� 0���ٸ÷�ӦΪ���ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���Ksp���ڸ��ݻ�ѧƽ���ƶ�ԭ�����μ�����Ũ���ᣬ������Ӧ��A2����H�� HA����A2��Ũ�ȼ�С��CaA���ܽ�ƽ�������ƶ���n(Ca2��)����������Һ����仯��������c(Ca2��)����2��CaA����Һ�м���CuSO4��Һ���������ֽⷴӦ������һ�ֺ�ɫ��������CuA�����ӷ���ʽΪCaA��s��+Cu2+��aq��?Ca2+��aq��+CuA��s�������Ҫ����Cu(OH)2����������c��Cu2+����c2��OH������Ksp[Cu(OH)2] ��2.0��10��20��c (Cu2��)��0.02 mol/L����c��OH������10��9��Ӧ������ҺpH��ʹ֮����5����3�������£���ij������Һ�е����̪����Һ�ʺ�ɫ�������Һ�ʼ��ԣ�ʵ�鷽��Ϊ������Ba2����ȥ̼������ӵĸ��ţ������Һ���Ժ�ɫ����֤����ͬѧ�Ĺ۵���ȷ���������̼���ˮ���Ե�ʣ�����ͬѧ�Ĺ۵���ȷ��
HA����A2��Ũ�ȼ�С��CaA���ܽ�ƽ�������ƶ���n(Ca2��)����������Һ����仯��������c(Ca2��)����2��CaA����Һ�м���CuSO4��Һ���������ֽⷴӦ������һ�ֺ�ɫ��������CuA�����ӷ���ʽΪCaA��s��+Cu2+��aq��?Ca2+��aq��+CuA��s�������Ҫ����Cu(OH)2����������c��Cu2+����c2��OH������Ksp[Cu(OH)2] ��2.0��10��20��c (Cu2��)��0.02 mol/L����c��OH������10��9��Ӧ������ҺpH��ʹ֮����5����3�������£���ij������Һ�е����̪����Һ�ʺ�ɫ�������Һ�ʼ��ԣ�ʵ�鷽��Ϊ������Ba2����ȥ̼������ӵĸ��ţ������Һ���Ժ�ɫ����֤����ͬѧ�Ĺ۵���ȷ���������̼���ˮ���Ե�ʣ�����ͬѧ�Ĺ۵���ȷ��
���㣺����������Һ�������ܽ�ƽ�⡣

25 ��ʱ,����ƽ�ⳣ��:
| ����Ļ�ѧʽ | CH3COOH | HClO | H2CO3 |
| ����ƽ�ⳣ��(25 ��) | 1.8��10-5 | 3.0�� | K1=4.3��10-7 K2=5.6��10-11 |
�ش���������:
(1)���ʵ���Ũ��Ϊ0.1 mol/L��������������:
a.Na2CO3,b.NaClO,c.CH3COONa,d.NaHCO3;
pH�ɴ�С��˳��������������������(����)��
(2)������0.1 mol/L��CH3COOH��Һ��ˮϡ������,���б���ʽ������һ����С������������;
A.c(H+) B.c(H+)/c(CH3COOH) C.c(H+)��c(OH-) D.c(OH-)/c(H+)
(3)���Ϊ10 mL pH=2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1 000 mL,ϡ����pH�仯��ͼ,��HX�ĵ���ƽ�ⳣ����������(����ڡ��������ڡ���С�ڡ�)�����ƽ�ⳣ��;�������� ��,
ϡ�ͺ�,HX��Һ��ˮ���������c(H+)��������(����ڡ��������ڡ���С�ڡ�)������Һ��ˮ���������c(H+),�������� ;

(4)25 ��ʱ,CH3COOH��CH3COONa�Ļ����Һ,����û��ҺpH=6,����Һ��c(CH3COO-)-c(Na+)=������������������������(��ȷ��ֵ)��
ijͬѧ�ù�ҵ����ͭ�����������������ʣ��Ʊ�������CuSO4��5H2O��������������
�����ֲ����������ԣ���
I��ȡ��ҵ����ͭ���壬��ϡ�����ܽ⣬���ˡ�
II������Һ�еμ�H2O2��Һ���Լ��ȡ�
III����II����Һ�м���CuO��ĩ��pH��4��
IV��������У����ˣ���Һ��ϡ�����ữ��pH��1��
V������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����þ��塣
��֪�����������������������pH��Ksp��25�棩���±���
| ���� | Fe(OH)3 | Fe (OH)2 | Cu(OH)2 |
| ��ʼ����ʱpH | 2.7 | 7.6 | 4.7 |
| ��ȫ����ʱpH | 3.7 | 9.6 | 6.7 |
| Ksp | 4.0��10�C38 | 8.0��10�C16 | 2.2��10�C20 |
��1��II�з�����Ӧ�����ӷ���ʽ�� ��
��2��II�н�Fe2+����ΪFe3+��Ŀ���� ��
��3����K3[Fe(CN)6]�����軯�أ���֤II��Fe2+�Ƿ�ת����ȫ�������� ��
��4��III�з�����Ӧ�����ӷ���ʽ�� ��
ͨ������˵���ڴ������µ���Һ��Fe3+�Ƿ������ȫ________________________(��ʾ������Һ��ij����Ũ��С��1.0��10�C5 mol/Lʱ����Ϊ�����ӳ�����ȫ)��
��5��Ӧ�û�ѧƽ���ƶ�ԭ������IV�С���Һ��ϡ�����ữ����ԭ�� ��
��1��25��ʱ��ijNaCl��Һ��c(Cl�C)��1��10��4 mol��L�C1�������Һ��c(Na��)��c(OH��)��
��2��25��ʱ����0.1 mol��L�C1NaOH��Һ��0.06 mol��L�C1��H2SO4��Һ��������(���Ի�Ϻ�����ı仯)����������Һ��pH�� ��25��ʱ��pHֵΪ8��NaOH��Һ��pHֵΪ10��NaOH��Һ�������Ϻ���Һ��������Ũ����ӽ� ��
��3��25��ʱ������������Һ�У���pH=0������ ��0.1 mol��L�C1������ ��0.01 mol��L�C1��NaOH��Һ ��pH=11��NaOH��Һ����ˮ��������������Ũ��֮�Ȣ٩U�کU�۩U���ǣ� (����ĸ)
| A��1�U10�U100�U1000 | B��0�U1�U12�U11 |
| C��14�U13�U12�U11 | D��14�U13�U2�U3 |
������¶���ˮ��Kw�� ��
���ڸ��¶��²��ij��ҺpH��3�������Һ��c(H��)��c(OH��)��________��
�۸��¶��½�pH=2�������pH=11������������Һ�������ϣ�pH=______________
��5�� ��ˮ��c(H+)=5.0��10�C7 mol��L�C1�����ʱ��ˮ�е�c(OH�C) = �����¶Ȳ��䣬����ϡ����ʹc(H+)=5.0��10�C3 mol��L�C1����c(OH�C) = ���ڸ��¶�ʱ����ˮ�е���NaOH��Һ����Һ�е�c(OH�C)=5.0��10�C2 mol��L�C1������Һ��c(H+)= ��
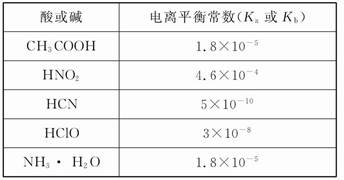
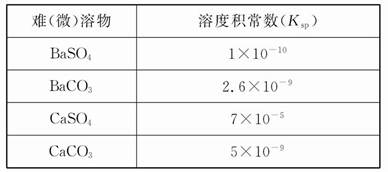
 CH3COO��+H+ ��H��0
CH3COO��+H+ ��H��0

 ��ֵ �����������С�����䡱����
��ֵ �����������С�����䡱���� mMn+��aq�� + nAm����aq����Ksp��[c��Mn+��]m��[c��Am����]n��
mMn+��aq�� + nAm����aq����Ksp��[c��Mn+��]m��[c��Am����]n��