��Ŀ����
����Ŀ����ҵ���������·�Ӧ��ȡP4��2Ca(PO4)2 +6SiO2+10C![]() 6CaSiO3+P4+10CO
6CaSiO3+P4+10CO
�ش��������⣺
(1)��̬��ԭ�ӵĺ�������Ų�ʽΪ___��
(2)Si��P��SԪ�ص�һ�����ܴ�С��ϵΪ___��
(3)P4��Pԭ�ӵ��ӻ���ʽ��___ ��P4�Ŀռ�ṹΪ___ �����ǡ�PPP=___��
(4)��CO��Ϊ�ȵ��ӵ���������___ (�ѧʽ)��
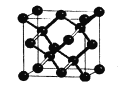
(5)���������ʯ�ṹ���ƣ���ͼΪ�����ľ����ṹ����֪��ԭ�ӵİ뾶Ϊr nm���������ܶ���___g/cm3��
(6)��ĺ��������ﶼ�Թ���������(SiO4)��Ϊ�����ṹ��Ԫ����ͼa��ʾ���ɼ�Ϊͼb��

�衢��ԭ��ͨ��������ԭ���γɸ��ֲ�ͬ�Ĺ���������ӣ���ͼc��ͼd��ͼc�Ļ�ѧʽ____________�����������Ĺ�����й���ԭ��֮��Ϊ____��

�衢��ԭ�ӳ����γɳ����⣬Ҳ���γɲ�״��������״�ṹ����������״�ṹ�У��衢��ԭ����֮��Ϊ____��
���𰸡�1s22s22p63s23p2����[Ne]3s23p2 P>S>Si��Si<S<P sp3 �������� 60�� CN�� ![]() Si2O76�� 1��3 1��2
Si2O76�� 1��3 1��2
��������
��1��Siλ�ڵ�������IVA�壬14��Ԫ�أ���������Ų�ʽΪ1s22s22p63s23p2����[Ne]3s23p2��
��2��ͬ���ڴ������ҵ�һ����������IIA>IIIA��VA>VIA����˵�һ�����ܴ�С˳����P>S>Si��Si<S<P��
��3�����Ŀռ乹��Ϊ���������Σ���Pԭ�ӵ��ӻ���ʽ��sp3������Ϊ60�㣻
��4��CO�ļ۵�������Ϊ10��ԭ����Ϊ2�����N2��NO����CN������CO��Ϊ�ȵ����壬��������CN����
��5�����������ʯ�ṹ���ƣ���Խ��ߵ�1/4=2r������Խ���Ϊ8r���ó������ı߳�Ϊ![]() ��10��7cm�����ݾ����Ľṹ�������к���Siԭ�Ӹ���Ϊ8����˾���������Ϊ
��10��7cm�����ݾ����Ľṹ�������к���Siԭ�Ӹ���Ϊ8����˾���������Ϊ![]() ��28g����˾������ܶ�Ϊ
��28g����˾������ܶ�Ϊ![]() g/cm3��
g/cm3��
��6����ͼ��֪��aͼ�Ļ�ѧʽΪSiO44�����γɶ��۹����������SiO44��ͨ������1��Oԭ����������֪ͼc�Ļ�ѧʽΪSi2O76��������ͼd�ṹ��ÿ��SiO44��������SiO44��������1��Oԭ�ӣ�Si��Oԭ�Ӹ�����Ϊ1��(2��2��1/2)=1��3����������״�ṹ�У�ÿ��SiO44�����ĸ�SiO44��������1��Oԭ�ӣ�Si��Oԭ�Ӹ�����Ϊ1��(4��1/2)=1��2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���ס��ҡ����������졢�������л���Ļ�ѧʽ���±���ʾ��
���� | �� | �� | �� | �� | �� | �� |
��ѧʽ | CH4 | C2H4 | C3H8 | C4H8 | C2H6O | C2H4O2 |
��������Щ�л����йص�˵������ȷ����
A. �ס���������ʹ���Ը��������Һ��ɫB. ���Ľṹ������5��(�����������칹)
C. ���������ҡ�����ȫȼ��ʱ���������D. �����ܷ���������Ӧ��������ˮ�ⷴӦ








