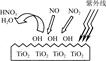
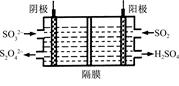
��Ŀ����
������ԭ��Ӧ�������������о��й㷺��;���ᴩ�Ž�
��1�����������������е��������з���������ԭ��Ӧ���� ������������ȷѡ�
| A���Ŵ�����ӡˢ | B��ԭ��ع��� | C����е֯�� | D���ҹ��Ŵ�ʪ����ͭ |
��д����ˮ�μӵķ��Ϸ�Ӧ���͢���һ����ѧ����ʽ�� ����ˮΪ ����
��3���Ȼ�麟����ں��ӡ��磺�ں���ͭ��ʱ���Ȼ�麟�ȥͭ�����������ͭ�Ա㺸�ӣ��䷴ӦΪ�� CuO+ NH4Cl = Cu+ CuCl2+ N2+ H2O
����ƽ��������ԭ��Ӧ����ʽ
�ڸ÷�Ӧ�У���������Ԫ���� ����Ԫ�����ƣ����������� ���ѧʽ��
�۷�Ӧ��������0.2mol�����壬���� ������ת�ơ�
13.��9�֣���1�� BD ��1�֣���ѡ����ѡ���÷֣���2�� 2Na + H2O =" 2NaOH" + H2 ����2�֣��� ���� ��1�֣������������𰸲��ո��֣���3����4 2 3 1 1 4��2�֣�ϵ��Ϊ1�����һ�֣��� �� ��1�֣��� CuO ��1�֣���1.2NA/7.2��1023��1�֣�
���������������1��������Ԫ�ػ��ϼ������ķ�Ӧ����������ԭ��Ӧ������ѡ��BD�ж��漰������ԭ��Ӧ��ѡ��AC�������仯��һ������������ԭ��Ӧ����ѡBD����2�������û���Ӧ����˷��������Ŀ�����C��H2O(g) CO��H2���ڸ÷�Ӧ����Ԫ�صĻ��ϼ۽��ͣ�����ˮ������������3��������ͭ���������ԣ��������Ȼ�李��ڷ�Ӧ��ͭ�Ļ��ϼ۴ӣ�2�۽��͵�0�ۣ��õ�2�����ӡ���Ԫ�صĻ��ϼ۴ӣ�3�����ߵ�0�ۣ�ʧȥ3�����ӣ������������ͻ�ԭ�������ʵ���֮����3�U2����˷�Ӧ�ķ���ʽΪ4CuO��2NH4Cl
CO��H2���ڸ÷�Ӧ����Ԫ�صĻ��ϼ۽��ͣ�����ˮ������������3��������ͭ���������ԣ��������Ȼ�李��ڷ�Ӧ��ͭ�Ļ��ϼ۴ӣ�2�۽��͵�0�ۣ��õ�2�����ӡ���Ԫ�صĻ��ϼ۴ӣ�3�����ߵ�0�ۣ�ʧȥ3�����ӣ������������ͻ�ԭ�������ʵ���֮����3�U2����˷�Ӧ�ķ���ʽΪ4CuO��2NH4Cl 3Cu��CuCl2��N2����4H2O���ڵ�Ԫ�صĻ��ϼ����ߣ����Ա�������Ԫ���ǵ���ͭԪ�صĻ��ϼ۽��ͣ��������ͭ�����������۸��ݷ���ʽ��֪��ÿ����1mol������ת��6mol���ӣ�����������0.2 mol�����壬����1.2mol�������ɡ�ת�Ƶ�����Ϊ1.2NA/7.2��1023��
3Cu��CuCl2��N2����4H2O���ڵ�Ԫ�صĻ��ϼ����ߣ����Ա�������Ԫ���ǵ���ͭԪ�صĻ��ϼ۽��ͣ��������ͭ�����������۸��ݷ���ʽ��֪��ÿ����1mol������ת��6mol���ӣ�����������0.2 mol�����壬����1.2mol�������ɡ�ת�Ƶ�����Ϊ1.2NA/7.2��1023��
���㣺����������ԭ��Ӧ���жϺ���ƽ�͵������ļ��㡣

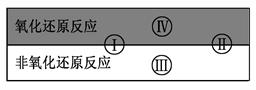
��֪������ԭ��Ӧ�����ֻ�����Ӧ���͵Ĺ�ϵ������ͼ��ʾ����������ˮ�μӻ����ɵļ��ַ�Ӧ��
��CaO+H2O =Ca(OH)2
��2Na+H2O=2NaOH+H2��
��H2+CuO  Cu +H2O
Cu +H2O
��3S+6NaOH  2Na2S +Na2SO3 +3H2O
2Na2S +Na2SO3 +3H2O
��NaOH+HCl=NaCl+H2O
��ش��������⣺
��1����Ӧ����ˮ ������ĸ����
| A���������� |
| B���ǻ�ԭ�� |
| C���������������ǻ�ԭ�� |
| D���Ȳ����������ֲ��ǻ�ԭ�� |
��3��������Ӧ�У������������� ������ţ���
��4��д��һ�ַ���������Ҽ��г�����������ˮ���ɵ����ӷ���ʽ�� ��
ŷ��ԭ����2012��1��1�������պ���̼��˰��Ӧ�Ա����ڻ���ȫ���ů��ʹ�ö���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������̼��Դ���о��Եø��ӽ��ȡ������û�ѧ��Ӧԭ�������֪ʶ�о�̼���仯��������ʡ�
��1�����������ҹ���������̼���о�ȡ���ش��չ���õ绡���ϳɵ�̼�����г����д���̼�����������ʣ�������̼���������������������ᴿ���䷴Ӧ�Ļ�ѧ����ʽΪ��
__C+__K2Cr2O7+__ ��__CO2��+ __K2SO4 + __Cr2(SO4)3+__H2O
����ɲ���ƽ������ѧ����ʽ��
��2���״���һ������ȼ�ϣ��״�ȼ�ϵ�ؼ�����ʵ��������ҵ����������ҵ��һ����CO��H2Ϊԭ�Ϻϳɼ״����÷�Ӧ���Ȼ�ѧ����ʽΪ��CO��g��+ 2H2��g�� CH3OH��g�� ��H1����116 kJ��mol-1
CH3OH��g�� ��H1����116 kJ��mol-1
�����д�ʩ������������÷�Ӧ�ķ�Ӧ���ʵ��� _______��
| A����ʱ��CH3OH�뷴Ӧ�������� | B�����ͷ�Ӧ�¶� |
| C��������ϵѹǿ | D��ʹ�ø�Ч���� |
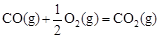 ��H2����283 kJ��mol-1
��H2����283 kJ��mol-1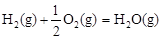 ��H3����242 kJ��mol-1
��H3����242 kJ��mol-1���ʾ1mol��̬�״���ȫȼ������CO 2��ˮ����ʱ���Ȼ�ѧ����ʽΪ ��
�����ݻ�Ϊ1L�ĺ��������У��ֱ��о���230�桢250���270�������¶��ºϳɼ״��Ĺ��ɡ���ͼ�����������¶��²�ͬ��H2��CO����ʼ��ɱȣ���ʼʱCO�����ʵ�����Ϊ1mol����COƽ��ת���ʵĹ�ϵ����ش�:
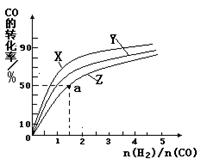
���������������¶��У�����Z��Ӧ���¶���
��������ͼ��a���Ӧ�����ݣ����������Z�ڶ�Ӧ�¶���CO��g��+ 2H2��g��
 CH3OH��g����ƽ�ⳣ��K = ��
CH3OH��g����ƽ�ⳣ��K = �� ˮ��������������Ҫ���ʣ��й�ˮ�ķ�Ӧʵ���кܶࡣ
(1)�������뽫̫����ת��Ϊ���ܣ�������ˮ�������������������һ�������Դ����������ˮ�Ĺ����У�ˮ��_____________(�����������������ԭ���ȱ������ֱ���ԭ���Ȳ��������ֲ�����ԭ)
(2)ҰӪ������Я���⻯�ƹ�����Ϊ�����������ʽΪCaH2+2H2O=Ca(OH)2+2H2��,����ˮ��_____________(�����������������ԭ���ȱ������ֱ���ԭ���Ȳ��������ֲ�����ԭ)
(3)�����з�Ӧ��ˮֻ����ԭ����_____________������ţ�
| A��C+H2O=CO+H2 | B��CaO+H2O=Ca(OH)2 |
| C��3Fe+4H2O=Fe3O4+4H2 | D��3NO2+H2O=2HNO3+NO |
3NO2+H2O=2HNO3+NO
 ��CO2+��K2SO4+��MnSO4+��H2O
��CO2+��K2SO4+��MnSO4+��H2O
 HNO3��д��OH��NO��Ӧ�Ļ�ѧ����ʽ��_____________���÷�Ӧ�б�������Ԫ���� ��
HNO3��д��OH��NO��Ӧ�Ļ�ѧ����ʽ��_____________���÷�Ӧ�б�������Ԫ���� ��