��Ŀ����
2�� �ü��顢�״����Ҵ������ѵȿ����Ƴ�ȼ�ϵ�أ�ʵ�����У����Լ״�ȼ�ϵ��Ϊֱ����Դ����һ��Ũ�ȵ���ȩ�� Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̣���ԭ������ȩ�ֱ�����������������Ӧ��ת��Ϊ�Ҵ������ᣬ��װ��ʾ��ͼ��ͼ��ʾ��
�ü��顢�״����Ҵ������ѵȿ����Ƴ�ȼ�ϵ�أ�ʵ�����У����Լ״�ȼ�ϵ��Ϊֱ����Դ����һ��Ũ�ȵ���ȩ�� Na2SO4��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̣���ԭ������ȩ�ֱ�����������������Ӧ��ת��Ϊ�Ҵ������ᣬ��װ��ʾ��ͼ��ͼ��ʾ����ȼ�ϵ����b��Ӧͨ��CH3OH����ṹ��ʽ�����壮
�ڵ������У�ijʱ����������Һ��Na2SO4��CH3COOH�����ʵ�����ͬ�������й��ڸ�ʱ����������Һ�и���Ũ�ȹ�ϵ��˵������ȷ����d������ĸ��ţ���
a��c��Na+����һ����c��SO42-����2��
b��c��Na+��=2c��CH3COOH��+2c��CH3COO-��
c��c��Na+����c ��CH3COOH����c��CH3COO-����c ��OH-��
d����Na+��+c��H+��=c��SO42-��+c��CH3COO-��+c��OH-��
���� ��bΪԭ��صĸ���������������Ӧ��Ӧͨ��CH3OH��
��a�������ӽ���Ĥ�ĽǶ��жϣ�
b�����������غ��жϣ�
c���ӵ���غ�ĽǶȷ�����
d������Ϊ������ʣ���Ҫ�Է����γɴ��ڣ�
��� �⣺��bΪԭ��صĸ���������������Ӧ��Ӧͨ��CH3OH���ʴ�Ϊ��CH3OH��
��a�������Ӿ������������ӽ���Ĥ��������������������Ӳ��ܾ��������ӽ���Ĥ����c��Na+����һ����c��SO42-����2������a��ȷ��
b��Na2SO4��CH3COOH�����ʵ�����ͬ������c��Na+��=2c��CH3COOH��+2c��CH3COO-������b��ȷ��
c������Ϊ������ʣ���Ҫ�Է����γɴ��ڣ�����c��Na+����c��CH3COOH����c��CH3COO-����c��OH-������c��ȷ��
d����ɲ��غ㣬ӦΪc��Na+��+c��H+��=2c��SO42-��+c��CH3COO-��+c��OH-������d����
�ʴ�Ϊ��d��
���� �����ڵ绯ѧ�Ļ������ۺϿ���ԭ��غ͵��صķ�Ӧԭ�������ʵķ����֪ʶ����Ŀ��Ϊ�ۺϣ��Ѷ��еȣ�ע����բ��������˼·�ͽǶȣ�

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ
12���±��г���A-R����Ԫ�������ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ��գ�
��1�������Ԫ���У�
��ѧ��������õ���Ar��
��������ǿ����K��
����������ˮ���������ǿ�ļ���KOH��
����������ˮ����������ǿ������HClO4��
��2��DԪ�ص�����������Ӧ��ˮ����������������Һ��Ӧ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��
��3��A��B��C����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳�����е�ΪK��Na��Mg��
��4��F���⻯��ĵ���ʽ ��G��H �⻯����ȶ��Եݼ���˳����HCl��HBr��
��G��H �⻯����ȶ��Եݼ���˳����HCl��HBr��
��5��HԪ�ظ�AԪ���γɵĻ�����Ļ�ѧʽ��NaBr��A2F2�ĵ���ʽ�� ����
����
��6��B��F����Ԫ���γɵ�2��1�ͻ������������ӻ��������ӻ�������ۻ�����������õ���ʽ��ʾ���γɹ��� ��
��
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | E | F | ||||||
| 3 | A | C | D | G | R | |||
| 4 | B | H |
��ѧ��������õ���Ar��
��������ǿ����K��
����������ˮ���������ǿ�ļ���KOH��
����������ˮ����������ǿ������HClO4��
��2��DԪ�ص�����������Ӧ��ˮ����������������Һ��Ӧ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��
��3��A��B��C����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳�����е�ΪK��Na��Mg��
��4��F���⻯��ĵ���ʽ
 ��G��H �⻯����ȶ��Եݼ���˳����HCl��HBr��
��G��H �⻯����ȶ��Եݼ���˳����HCl��HBr����5��HԪ�ظ�AԪ���γɵĻ�����Ļ�ѧʽ��NaBr��A2F2�ĵ���ʽ��
 ����
������6��B��F����Ԫ���γɵ�2��1�ͻ������������ӻ��������ӻ�������ۻ�����������õ���ʽ��ʾ���γɹ���
 ��
��
17�������йػ�ѧ������ȷ���ǣ�������
| A�� | Cl-���ӵĽṹʾ��ͼ�� | B�� | ����ĽṹʽΪ�� | ||
| C�� | ������ӵı���ģ��ʾ��ͼ�� | D�� | �Ҵ��ķ���ʽ��CH3CH2OH |
14������˵���У���ȷ���ǣ�������
| A�� | 22.4 L�����У�һ������2mol��ԭ�� | |
| B�� | 80 g NaOH�ܽ���1 Lˮ�У��õ���Һ�����ʵ���Ũ��Ϊ2mol/L | |
| C�� | 18 gˮ�ڱ�״���µ����ԼΪ22.4L | |
| D�� | ��״���£�20 mLNH3��60 mLO2�������Ӹ�����Ϊ1��3 |
12��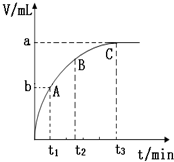 ijͬѧ��ʵ���ҽ���ʵ���о���ѧ��Ӧ���ʵ�Ӱ�����أ�
ijͬѧ��ʵ���ҽ���ʵ���о���ѧ��Ӧ���ʵ�Ӱ�����أ�
���±����о���ѧϰС���о�Ӱ��������⣨H2O2���ֽ����ʵ�����ʱ�ɼ���һ�����ݣ�
��10mL H2O2��Һ��ȡ150mL���������ʱ�䣨��λ��s��
��������ش�
��1����������ֽ�Ļ�ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��2�����о�С������Ʒ���ʱ��������Ũ�ȡ��¶ȡ������ȷ�Ӧ�����Թ�������ֽ����ʵ�Ӱ�죮
��3��������Ӱ���������ֽ����ʵ���������ѡһ����˵�������ضԷֽ������к�Ӱ�죿�¶�����ѧ��Ӧ���ʼӿ죨��Ӧ��Ũ������ѧ��Ӧ���ʼӿ죻ʹ�ú��ʵĴ�����ѧ��Ӧ���ʼӿ죩��
��4��ijͬѧ��10mL H2O2 ��Һ�м���һ�����Ķ������̣��ų�������������״�����뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����A��B��C��������ʾ�ļ�ʱ��Ӧ������������C��
��5����������MnO2�����ữ��H2O2����Һ�У�MnO2�ܽ����Mn2+����Ӧ�����ӷ���ʽΪMnO2+2H++H2O2=Mn2++O2��+2H2O��
�����ṩ����ʵ����Ʒ�о���ѧ��Ӧ���ʵ�Ӱ�����أ�
ʵ���������Թܡ��ձ�����Ͳ����ƽ�����
ʵ��ҩƷ����Ƭ�����ۡ�0.5mol/L ���ᡢ5mol/L ����
���������ṩʵ����Ʒ�����о��Ļ�ѧ��Ӧ���ʵ�Ӱ�������������Ũ�ȡ����ı������
Ϊ�˱��ڹ۲죬�㽨���¼�������Ƿ�Ӧ��ʼ��ֹͣ���õ�ʱ�䣮
����Ҫ�о��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬�����õ�����Ҫ�������¶ȼƣ�
 ijͬѧ��ʵ���ҽ���ʵ���о���ѧ��Ӧ���ʵ�Ӱ�����أ�
ijͬѧ��ʵ���ҽ���ʵ���о���ѧ��Ӧ���ʵ�Ӱ�����أ����±����о���ѧϰС���о�Ӱ��������⣨H2O2���ֽ����ʵ�����ʱ�ɼ���һ�����ݣ�
��10mL H2O2��Һ��ȡ150mL���������ʱ�䣨��λ��s��
Ũ �� ��Ӧ���� | 30% H2O2 | 15% H2O2 | 10% H2O2 | 5% H2O2 |
| ������������ | ��������Ӧ | ��������Ӧ | ��������Ӧ | ��������Ӧ |
| �������� �� | 360 | 480 | 540 | 720 |
| MnO2���������� | 10 | 25 | 60 | 120 |
��1����������ֽ�Ļ�ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_{2}\;}}{\;}$2H2O+O2����
��2�����о�С������Ʒ���ʱ��������Ũ�ȡ��¶ȡ������ȷ�Ӧ�����Թ�������ֽ����ʵ�Ӱ�죮
��3��������Ӱ���������ֽ����ʵ���������ѡһ����˵�������ضԷֽ������к�Ӱ�죿�¶�����ѧ��Ӧ���ʼӿ죨��Ӧ��Ũ������ѧ��Ӧ���ʼӿ죻ʹ�ú��ʵĴ�����ѧ��Ӧ���ʼӿ죩��
��4��ijͬѧ��10mL H2O2 ��Һ�м���һ�����Ķ������̣��ų�������������״�����뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ����A��B��C��������ʾ�ļ�ʱ��Ӧ������������C��
��5����������MnO2�����ữ��H2O2����Һ�У�MnO2�ܽ����Mn2+����Ӧ�����ӷ���ʽΪMnO2+2H++H2O2=Mn2++O2��+2H2O��
�����ṩ����ʵ����Ʒ�о���ѧ��Ӧ���ʵ�Ӱ�����أ�
ʵ���������Թܡ��ձ�����Ͳ����ƽ�����
ʵ��ҩƷ����Ƭ�����ۡ�0.5mol/L ���ᡢ5mol/L ����
���������ṩʵ����Ʒ�����о��Ļ�ѧ��Ӧ���ʵ�Ӱ�������������Ũ�ȡ����ı������
Ϊ�˱��ڹ۲죬�㽨���¼�������Ƿ�Ӧ��ʼ��ֹͣ���õ�ʱ�䣮
����Ҫ�о��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬�����õ�����Ҫ�������¶ȼƣ�

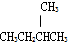 D��
D�� ��
�� E��CH3CH2CH2CH3��
E��CH3CH2CH2CH3��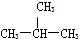




 ��1�������Ͻ����κ��Է���������ԭ��Ӧ��������Ƴ�ԭ��أ������÷�Ӧ��Cu+2Ag+=2Ag+Cu2+������һ����ѧ��أ�����������̼�������ش��������⣺
��1�������Ͻ����κ��Է���������ԭ��Ӧ��������Ƴ�ԭ��أ������÷�Ӧ��Cu+2Ag+=2Ag+Cu2+������һ����ѧ��أ�����������̼�������ش��������⣺