��Ŀ����
6�����������У���1����Ϊͬ���칹�����FI
��2����Ϊͬ�����������AD
��3������ͬλ�ص���BC
��4������ͬһ�����ʵ���G�����ţ�
A�����ʯ��ʯī B��${\;}_{20}^{40}K$��${\;}_{20}^{40}$Ca C��${\;}_{6}^{12}$C��${\;}_{6}^{14}$C D��O2��O3
E��SO2��SO3 F��CH3CH2OH��CH3OCH3 G��D2O��T2O
H��
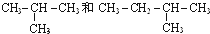 I��
I��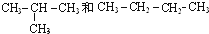
���� ��1������ʽ��ͬ���ṹ��ͬ���л���֮�以Ϊͬ���칹�壻
��2��ͬ����������ͬ��Ԫ�صIJ�ͬ����֮��Ļ��ƣ�
��3��������ͬ���������Ͳ�ͬ��������ͬ��Ԫ�صIJ�ͬԭ��֮�以Ϊͬλ�أ�
��4��������ͬ�ķ���ʽ����ͬ�Ľṹ����������ͬһ�����ʣ�
��� �⣺��1��CH3CH2OH��CH3OCH3��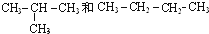 �Ƿ���ʽ��ͬ���ṹ��ͬ���л����Ϊͬ���칹�壬�ʴ�Ϊ��FI��
�Ƿ���ʽ��ͬ���ṹ��ͬ���л����Ϊͬ���칹�壬�ʴ�Ϊ��FI��
��2�����ʯ��ʯī��ͬ��̼Ԫ����ɵIJ�ͬ���ʣ���Ϊͬ�������壻O2��O3��ͬ����Ԫ����ɵIJ�ͬ���ʣ���Ϊͬ�������壬�ʴ�Ϊ��AD��
��3��${\;}_{20}^{40}K$��${\;}_{20}^{40}$Ca�Ǿ�����ͬ������������ͬ�������ĸ�Ԫ�صIJ�ͬԭ�ӣ���Ϊͬλ�أ�${\;}_{6}^{12}$C��${\;}_{6}^{14}$C�Ǿ�����ͬ������������ͬ��������̼Ԫ�صIJ�ͬԭ�ӣ���Ϊͬλ�أ��ʴ�Ϊ��BC��
��4��D2O��T2O����ˮ���ӣ�����ͬһ�����ʣ��ʴ�Ϊ��G��
���� ���⿼�����ı��������ء���ͬ������Ŀ��飬���ո����Ҫ�㼰��������Ϊ���Ĺؼ������ڻ���֪ʶ��ѵ������Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ
16���������[KMnO4]�dz��õ�����������ҵ�������̿���Ҫ�ɷ���MnO2��Ϊԭ���Ʊ�������ؾ��壮�м����Ϊ�����[K2MnO4]����ͼ1��ʵ����ģ���Ʊ��IJ������̣�
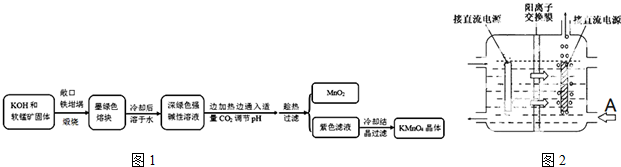
������ϣ�
�������ܽ��
�������[K2MnO4]�����״��ī��ɫ�ᾧ����ˮ��Һ������ɫ�������������MnO42-����������ɫ��
��ѧ���ʣ���ǿ������Һ���ȶ��������ԡ����Ժ������Ի����£�MnO42-�ᷢ���绯��Ӧ��
�Իش��������⣺
��1���������̿��KOH����ʱ��������ʯӢ������ѡ���������������Ǹ�����ǿ���ʹ������еĶ������跴Ӧ��ʴ������ʵ���������������ձ�¶�ڿ����еĹ����������Ӧ�Ļ�ѧ����ʽΪ2MnO2+4KOH+O2$\frac{\underline{\;����\;}}{\;}$2K2MnO4+2H2O��
��2��ʵ��ʱ����CO2����������KHCO3�����µõ���KMnO4��Ʒ�Ĵ��Ƚ��ͣ���д��ʵ����ͨ������CO2ʱ��ϵ�п��ܷ�����Ӧ���ӷ���ʽ��3MnO42-+2CO2�T2MnO4-+MnO2��+2CO32-��2OH-+CO2�TCO32-+H2O������������ԭ��Ӧ���������ͻ�ԭ����������Ϊ1��2��
��3������CO2��ͨ�������ѿ��ƣ���˶�����ʵ�鷽�������˸Ľ�������ʵ����ͨCO2��Ϊ���������ᣮ�������Ϸ�����ѡ����������A���õ��IJ�Ʒ���ȸ��ߣ�
A������ B��Ũ���� C��ϡ����
��4����ҵ��һ����ö��Ե缫����������Һ��ȡ������أ���д���õ�ⷴӦ�Ļ�ѧ����ʽ2K2MnO4+2H2O$\frac{\underline{\;����\;}}{\;}$2KMnO4+H2��+2KOH��
��ͳ���ղ�����Ĥ��ⷨ���ڸ���Ӧ������MnԪ�������ʺ͵���Ч�ʶ���ƫ�ͣ���ͬѧ���뵽���ӽ���Ĥ����ⱥ��ʳ��ˮ����Ľ����������������ӽ���Ĥ�ָ����������е�⣨��ͼ2����ͼ��A�ڼ������Һ���ΪKOH��Һ��ʹ�������ӽ���Ĥ�������MnԪ�������ʵ�ԭ��Ϊ�����ӽ���Ĥ��ֹ�������������������ԭ��

������ϣ�
�������ܽ��
| ���� | KMnO4 | K2CO3 | KHCO3 | K2SO4 | CH3COOK |
| 20���ܽ�� | 6.4 | 111 | 33.7 | 11.1 | 217 |
��ѧ���ʣ���ǿ������Һ���ȶ��������ԡ����Ժ������Ի����£�MnO42-�ᷢ���绯��Ӧ��
�Իش��������⣺
��1���������̿��KOH����ʱ��������ʯӢ������ѡ���������������Ǹ�����ǿ���ʹ������еĶ������跴Ӧ��ʴ������ʵ���������������ձ�¶�ڿ����еĹ����������Ӧ�Ļ�ѧ����ʽΪ2MnO2+4KOH+O2$\frac{\underline{\;����\;}}{\;}$2K2MnO4+2H2O��
��2��ʵ��ʱ����CO2����������KHCO3�����µõ���KMnO4��Ʒ�Ĵ��Ƚ��ͣ���д��ʵ����ͨ������CO2ʱ��ϵ�п��ܷ�����Ӧ���ӷ���ʽ��3MnO42-+2CO2�T2MnO4-+MnO2��+2CO32-��2OH-+CO2�TCO32-+H2O������������ԭ��Ӧ���������ͻ�ԭ����������Ϊ1��2��
��3������CO2��ͨ�������ѿ��ƣ���˶�����ʵ�鷽�������˸Ľ�������ʵ����ͨCO2��Ϊ���������ᣮ�������Ϸ�����ѡ����������A���õ��IJ�Ʒ���ȸ��ߣ�
A������ B��Ũ���� C��ϡ����
��4����ҵ��һ����ö��Ե缫����������Һ��ȡ������أ���д���õ�ⷴӦ�Ļ�ѧ����ʽ2K2MnO4+2H2O$\frac{\underline{\;����\;}}{\;}$2KMnO4+H2��+2KOH��
��ͳ���ղ�����Ĥ��ⷨ���ڸ���Ӧ������MnԪ�������ʺ͵���Ч�ʶ���ƫ�ͣ���ͬѧ���뵽���ӽ���Ĥ����ⱥ��ʳ��ˮ����Ľ����������������ӽ���Ĥ�ָ����������е�⣨��ͼ2����ͼ��A�ڼ������Һ���ΪKOH��Һ��ʹ�������ӽ���Ĥ�������MnԪ�������ʵ�ԭ��Ϊ�����ӽ���Ĥ��ֹ�������������������ԭ��
17�������й����ʵ�������Ӧ�����Ӧ���ǣ�������
| A�� | Ũ���������ˮ�ԣ������ڸ��������̼����������� | |
| B�� | ������������ˮ������������� | |
| C�� | Ũ�����ڳ�������ʹ���ۻ����������۳�����Ũ���� | |
| D�� | SO2����ǿ��ԭ�ԣ��ڿ����м��ױ�����ΪSO3 |
14�����и�������������ͬ���칹����ǣ�������
| A�� | H2O��D2O | B�� | �״��Ͷ�����CH3-O-CH3 | ||
| C�� | 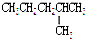 �� �� | D�� |  �� ��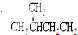 |
1����֪Ԫ�ص�ԭ��������������֪ԭ�ӵĢ�ԭ�����ں˵�����ۺ���������������ڱ��е�λ�ã�������ȷ���ǣ�������
| A�� | �٢� | B�� | �ڢ� | C�� | �٢ڢ� | D�� | �ڢۢ� |
11�������л��������Դ������·���ˮ�ⷴӦ���������ֲ�ͬ���л�����������л������Է���������ȣ����л����ǣ�������
| A�� | ���ᶡ�� | B�� | ������ | C�� | ������� | D�� | �������� |
18����λͬѧ��һ������ij�����ʣ���������ǵ������жϸ������ǣ�������


| A�� | Na2CO3 | B�� | CaCO3 | C�� | CaCl2 | D�� | NaHCO3 |
15���������ʿ���ʹ�����ʱ��Ե��ǣ���������
�ٸ������� �ھƾ� ������������ �ܣ�NH4��22SO4 ��CuSO4 ��˫��ˮ �����ᣮ
�ٸ������� �ھƾ� ������������ �ܣ�NH4��22SO4 ��CuSO4 ��˫��ˮ �����ᣮ
| A�� | ������ | B�� | ���ۡ����� | C�� | �٢ڢ� | D�� | ���ܡ����� |
16������˵������ȷ���ǣ�������
| A�� | �������������ԭ�ӽ�����̬ԭ�� | |
| B�� | 3s2��ʾ3s�ܼ���������� | |
| C�� | ͬһԭ���У�1s��2s��3s���ӵ�������С | |
| D�� | ͬһԭ���У�3d��4d��5d�ܼ��Ĺ������������ |