��Ŀ����
����Ŀ����֪ij���巴Ӧ��ƽ�ⳣ���ɱ�ʾΪK=![]() ���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ����400�棬K=32��500�棬K=44����ش��������⣺
���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ����400�棬K=32��500�棬K=44����ش��������⣺
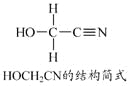
��1��д��������Ӧ�Ļ�ѧ����ʽ____��
��2����֪���ܱ������У����ijʱ�̸���ֵ�Ũ�����£�
���� | CH3OH(g�� | CH3OCH3(g�� | H2O(g�� |
Ũ��/��molL-1�� | 0.54 | 0.68 | 0.68 |
�ٴ�ʱ�¶�400�棬��ijʱ������____���������������ͬ����
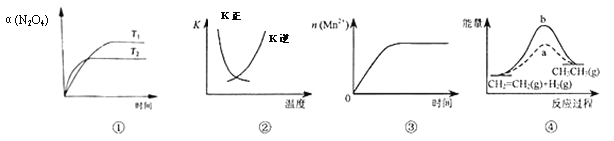
�������¶�Ϊ�����꣬�Ը��¶���ƽ��̬�״����ʵ���nΪ�����꣬��ʱ��Ӧ����ͼ���λ����ͼ��___�㣬�Ƚ�ͼ��B��D��������Ӧ������Ӧ������B_____��D��������____��

��3��һ��������Ҫ��߷�Ӧ���ת���ʣ����Բ��õĴ�ʩ��___��
a�������¶� b��������� c��ѹ����������� d������ˮ������Ũ�� e����ʱ���������
���𰸡�2CH3OH![]() CH3OCH3+H2O > A < �¶����ߣ���ѧ��Ӧ���ʼӿ� ae
CH3OCH3+H2O > A < �¶����ߣ���ѧ��Ӧ���ʼӿ� ae
��������
��1����ѧƽ�ⳣ����ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ����÷�Ӧ�ķ�Ӧ��Ϊ�״���������Ϊ�����Ѻ�ˮ��
��2�����¶�400��ʱ��ijʱ��Ũ����QcС��ƽ�ⳣ��������ƽ��������Ӧ�����ƶ���
��ƽ��������Ӧ�����ƶ����״��İٷֺ�������ƽ��ʱ�ĺ������¶�Խ�߷�Ӧ����Խ��
��3�����ݻ�ѧƽ���ƶ�ԭ�������жϡ�
��1����ƽ�ⳣ���ɱ�ʾΪK=![]() ��֪���÷�Ӧ�ķ�Ӧ��Ϊ�״���������Ϊ�����Ѻ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2CH3OH��g��
��֪���÷�Ӧ�ķ�Ӧ��Ϊ�״���������Ϊ�����Ѻ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2CH3OH��g��![]() CH3OCH3��g��+H2O��g�����ʴ�Ϊ��2CH3OH��g��
CH3OCH3��g��+H2O��g�����ʴ�Ϊ��2CH3OH��g��![]() CH3OCH3��g��+H2O��g����
CH3OCH3��g��+H2O��g����
��2�����¶�400��ʱ��ijʱ��Ũ����Qc=![]() =
=![]() =1.55��32����ƽ��������Ӧ�����ƶ���v����v�����ʴ�Ϊ��>��
=1.55��32����ƽ��������Ӧ�����ƶ���v����v�����ʴ�Ϊ��>��
���ɢٿ�֪ƽ��������Ӧ�����ƶ������Լ״��İٷֺ�������ƽ��ʱ�ĺ�������ʱ��Ӧ��Ӧ��A�㣻����Ӱ�컯ѧ��Ӧ���ʵ����أ��¶�Խ�߷�Ӧ����Խ��D���¶ȸ���B�㣬����D���Ӧ������Ӧ����Ҳ����B�㣬�ʴ�Ϊ��A�������¶����ߣ���ѧ��Ӧ���ʼӿ죻
��3��a������Ӧ�����ȷ�Ӧ�������¶�ƽ��������Ӧ�����ƶ����״���ƽ��Ũ�ȼ�С��ת����������ȷ��
b�����������ƽ�ⲻ�ƶ����״���ת���ʲ��䣬�ʴ���
c���÷�ӦΪ�����������ķ�Ӧ��ѹ�����������ѹǿ����ƽ�ⲻ�ƶ����״���ת���ʲ��䣬�ʴ���
d������ˮ����Ũ�ȣ�ƽ�����淴Ӧ�����ƶ����״���ת���ʼ�С���ʴ���
e����ʱ������ƽ��������Ӧ�����ƶ����״���ת����������ȷ��
ae��ȷ���ʴ�Ϊ��ae��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ƽ�ⳣ������Ka��ʾ)�Ĵ�С�����жϵ���ʵ����ǿ����25��ʱ���й����ʵĵ���ƽ�ⳣ�����±���ʾ�����ݸñ����ش��������⣺
��ѧʽ | HF | CH3COOH | H2SO3 | H2CO3 | H2S |
����ƽ�� ������Ka�� | 4.0��10-4 | 1.8��10-5 | K1=1.54��10-2 K2=1.02��10-7 | K1=4.4��10-7 K2=4.7��10-11 | K1��9.1��10��8 K2��1.1��10��12 |
(1)ͬŨ�ȵ�F����CO32-��CH3COO-��HS-���H����������ǿ��Ϊ__________
(2)��Һ�в����ܴ����������������__________
a��HS-��SO32- b��HF��CH3COO- c��HS-��HCO3- d. HSO3-��HCO3-
(3)Na2CO3��Һͨ�����H2S�����ӷ���ʽ�ǣ�_____��
(4)OH��Ũ����ͬ�ĵ������������ҺHCl��A���� CH3COOH��E�����ֱ���п�۷�Ӧ����������һ����Һ�д���п���ų�������������ͬ��������˵����ȷ����_______(��д���)��
�ٷ�Ӧ����Ҫ��ʱ��E>A �ڿ�ʼ��Ӧʱ������A>E
�۲μӷ�Ӧ��п�����ʵ���A��E �ܷ�Ӧ���̵�ƽ������E>A
��A��Һ����пʣ�� ��E��Һ����пʣ��
(5)�� 0.l molL-1 CH3COOH��Һ�еμ� NaOH ��Һ��c(CH3COOH) : c(CH3COO-) =2 : 36����ʱ��ҺpH = __________��