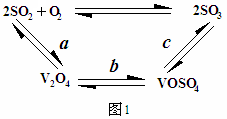
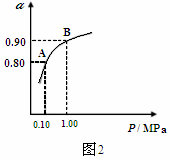
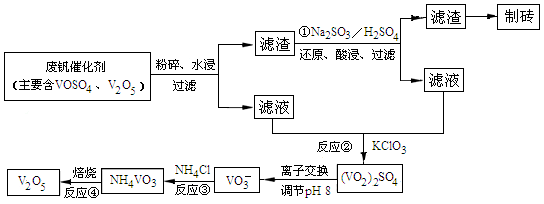
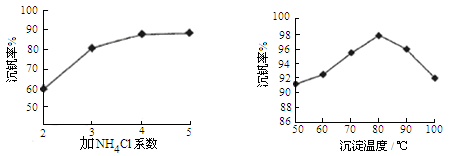
��Ŀ����
����ѧ����ѡ��2����ѧ�뼼������15�֣�
ͨ����ˮ�ܻ�õ�ˮ��ʳ�Ρ�þ�ȣ�ʳ�οɽ�һ�������ȼҵ����ش��������⡣
��1���о����ֺ�ˮ�����ķ�����_________��_________��
��2���ȼҵͨ����ⱥ��ʳ��ˮ�ܻ���ռ�����������ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ_____________����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ������ʯī�ӵ�Դ_________�������ʱ���缫�ĵ缫��ӦʽΪ_________����������ͨ�����ӽ���Ĥ����Ҫ������__________��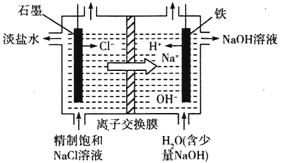
��3�������Ƽ�У�������ʳ��ˮ��ͨ��CO2��NH3�Ʊ�NaHCO3���仯ѧ����ʽΪ____________����ͨ�����__________���ѧʽ������������__________________ ��������NaHCO3���ȷֽ���Ʊ����
��4��Ŀǰ������60%���ϵ�þ���ǴӺ�ˮ����ȡ�ģ���֪��MgO��MgCl2���۵�ֱ�Ϊ2852���714�档����˵����ҵ�ϲ��õ������MgCl2�����ǵ������MgO������__________ ______________________________________________________________________ ��
��1���������ӽ�������������������ѡ��������2�֣�
��2��2NaCl+2H2O���2NaOH+H2��+Cl2����2�֣� ����1�֣� 2H++2e- H2����2�֣�
H2����2�֣�
Na+��H+��1�֣�
��3��NaCl+NH3+CO2+H2O NaHCO3+NH4Cl��2�֣� NH3��1�֣� NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С��2�֣�
NaHCO3+NH4Cl��2�֣� NH3��1�֣� NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С��2�֣�
��4�������۵�MgO��MgCl2�����MgO�ķѵ�࣬�����MgCl2�ĵ���٣�2�֣�
�������������
��1���������ӽ�������������������ѡ������
��2��2NaCl+2H2O���2NaOH+H2��+Cl2�� �� 2H++2e- H2��
H2��
Na+��H+
��3��NaCl+NH3+CO2+H2O NaHCO3+NH4Cl NH3 NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С
NaHCO3+NH4Cl NH3 NH3��������NaCl��Һ����CO2��NaCl��Һ���ܽ�Ⱥ�С
��4�������۵�MgO��MgCl2�����MgO�ķѵ�࣬�����MgCl2�ĵ����
���㣺���黯ѧ�������е�Ӧ�á�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д������йؽ����Ĺ�ҵ�Ʒ��У���ȷ���ǣ� ��
| A����ͭ���û�ͭ����Ҫ�ɷ�ΪCuFeS2��ֱ�ӵ�⾫���õ�����Ϊ99.9%��ͭ |
| B����������ҵ�ϵ���Ȼ������Ʊ��� |
| C�����ƣ���ⱥ��NaCl��Һ |
| D����������CO�ڸ����»�ԭ����ʯ�е��� |
���н���ͨ�����ü��ȷֽ�ķ�����ұ������ ( )
| A��Na | B��Al | C��Fe | D��Ag |
����˵���У�����ȷ����
| A���������ܴӺ�ˮ����ȡ��ˮ |
| B���Ӻ�ˮ�п��Եõ��Ȼ�þ���ټ��ȷֽ���ƽ���þ |
| C���������Ӻ�ˮ������Ĺؼ���Ӧ��Cl2+2Br��= 2Cl��+Br2 |
D��ú��������Ҫ��Ӧ�� |
�±��н�����ұ��ԭ���뷽������ȫ��ȷ���ǣ� ��
| | ұ��ԭ�� | ���� |
| A | 2HgO 2Hg��O2�� 2Hg��O2�� | �ȷֽⷨ |
| B | 2Al2O3�����ڣ� 4Al��3O2�� 4Al��3O2�� | ��ⷨ |
| C | Cu2S��O2 2Cu��SO2 2Cu��SO2 | �ȷֽⷨ |
| D | Fe2O3��2Al 2Fe��Al2O3 2Fe��Al2O3 | �Ȼ�ԭ�� |


 Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��
Ti+2MgCl2��ú���״�ѡ��÷�Ӧ���������ֻ����н���_______ (�����)��