��Ŀ����
����Ŀ����ͼΪ����������Ӧʵ��װ�ã�
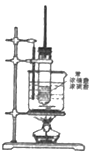
��1��д���÷�Ӧ����ʽ__________________________����Ӧ����Ϊ_____________________��
��2����Ӧ�в��õļ��ȷ�ʽΪ________________�������¶�Ϊ_______________���Թ��Ϸ������ܵ�������___________________________________��
��3�������йر�����������������ȷ����_______________��
a �ܶȶ���ˮС b ��������ˮ c ��Ϊͬϵ�� d �����ڷ�����
��4���л���Ӧ��Ҫ��Ϊȡ������������ȥ���ӳɡ��ۺ�������͡��������з�Ӧ��
������ϩ�������� �������ڿ�����ȼ�� ����ϩʹ��ˮ��ɫ����ϩͨ�����Ը��������Һ ������ϩ�ƾ���ϩ ����������������
��������ȡ����Ӧ����___________________������������Ӧ����_____________________��
���ڼӳɷ�Ӧ����___________________�����ھۺϷ�Ӧ����___________________��
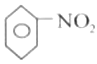
���𰸡�![]() +HO-NO2
+HO-NO2![]()
 +H2O ȡ����Ӧ ˮԡ���� 50����60�� �������� b ����ȡ����Ӧ������ ����������Ӧ�����ڢ� ���ڼӳɷ�Ӧ�����٢� ���ھۺϷ�Ӧ������
+H2O ȡ����Ӧ ˮԡ���� 50����60�� �������� b ����ȡ����Ӧ������ ����������Ӧ�����ڢ� ���ڼӳɷ�Ӧ�����٢� ���ھۺϷ�Ӧ������
��������
������Ӧ��ŨH2SO4�Ƿ�Ӧ�Ĵ�������ˮ������Ӧ���¶ȵ�Ҫ��ʮ���ϸ������Ҫˮԡ���ȣ�����¶ȳ���60�棬���ж���������������ȸ��������ɣ������£�����Ũ���ᶼ�ӷ���55��60��ӷ�������װ���еij����ܾ��������������ã�����������Ũ����ʹ�������������������ˮ����˿���ˮϴ�����롣
��1��![]() ��ŨH2SO4�����£���Ũ���Ṳ�ȷ���ȡ����Ӧ����
��ŨH2SO4�����£���Ũ���Ṳ�ȷ���ȡ����Ӧ����![]() ��ˮ����Ӧ�Ļ�ѧ����ʽΪ
��ˮ����Ӧ�Ļ�ѧ����ʽΪ![]() +HO-NO2
+HO-NO2![]()
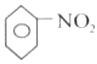 +H2O���ʴ�Ϊ��
+H2O���ʴ�Ϊ��![]() +HO-NO2
+HO-NO2![]()
 +H2O��ȡ����Ӧ��
+H2O��ȡ����Ӧ��
��2���÷�Ӧ���¶ȵ�Ҫ��ʮ���ϸ������Ҫˮԡ���ȣ�����¶ȳ���60�棬���ж���������������ȸ��������ɣ������£�����Ũ���ᶼ�ӷ���55��60��ӷ�������װ���еij����ܾ��������������ã�����������Ũ����ʹ��������ʴ�Ϊ��ˮԡ���ȣ�50����60�棻����������
��3��a�������ܶȱ�ˮС�����������ܶȱ�ˮ�ʴ���
b��������������������ˮ������ȷ��
c�����������������������������������Ϊͬϵ��ʴ���
d�������ڷ����������������ڷ��㻯����ʴ���
b��ȷ���ʴ�Ϊ��b��
��4������ϩ���Ȼ��ⷢ���ӳɷ�Ӧ���������飻
�������ڿ�����ȼ�����ɶ�����̼��ˮ�ķ�Ӧ����������Ӧ��
����ϩ������ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ��
����ϩ�����Ը��������Һ����������Ӧ��ʹ���Ը��������Һ��ɫ��
����ϩһ�������·����Ӿ۷�Ӧ���ɾ���ϩ��
�������������ڹ��������·���ȡ����Ӧ�����ȴ�����
������ȡ����Ӧ���Ǣޣ�����������Ӧ���Ǣڢܣ����ڼӳɷ�Ӧ���Ǣ٢ۣ����ھۺϷ�Ӧ���Ǣݣ��ʴ�Ϊ���ޣ��ڢܣ��٢ۣ��ݡ�