��Ŀ����
������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ����
| A��25��ʱ��pH=7��NH4Cl��NH3?H2O�����Һ��c��H+��=c��OH-��=c��NH4+��=c��Cl-�� |
| B��0.1mol/LNa2S��Һ��c��OH-��=c��H+��+c��HS-��+c��H2S�� |
| C��25��ʱ��pH=2��CH3COOH��pH=12��NaOH�������ϣ� c��CH3COO-��+c��H+����c��Na+��+c��OH-�� |
| D��0.1mol/LNa2CO3��Һ��2c��CO32-��+2c��HCO-��+2c��H2CO3��=c��Na+�� |
D
�������������A��������Һ����c��H+��=c��OH-�����ַ��ϵ���غ㣬c��H+��+ c��NH4+��= c��OH-��+ c��Cl-����������c��NH4+��=c��Cl-��> c��H+��=c��OH-��,����B��0.1mol/LNa2S��Һ�У����������غ��c��OH-��=c��H+��+c��HS-��+2c��H2S��������C��25��ʱ��pH=2��CH3COOH��pH=12��NaOH��Һ�У�c(CH3COOH)>c(NaOH),�������Ϻ����ҺʵΪCH3COOH��CH3COONa����c��CH3COO-��+c��H+��>c��Na+��+c��OH-��,����D������Ԫ���غ��2c��CO32- ��+2c��HCO- ��+2c��H2CO3��=��Na+������ȷ����ѡD��
���㣺������Һ������Ũ�ȵĴ�С��ϵ���غ���ɵ�Ӧ��

��֪����������K2SO4��MgSO4��2CaSO4��ˮ�д�������ƽ�⣺
K2SO4��MgSO4��2CaSO4(s)=2Ca2++2K++Mg2++4SO42-����ͬ�¶��£�K+�Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ����ͼ��ʾ��������˵���������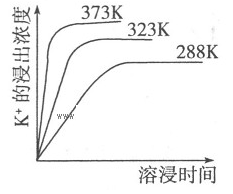
| A�������ϵ�м��뱥��NaOH��Һ���ܽ�ƽ�������ƶ� |
| B�������ϵ�м��뱥��̼������Һ���ܽ�ƽ�������ƶ� |
| C����ƽ���Ksp=c(Ca2+)��c(K+)��c(Mg2+)��c(SO42-) |
| D�������¶ȣ���Ӧ��������ƽ��������Ӧ�����ƶ� |
��Ca��OH��2��������ˮ�У�һ��ʱ���ﵽ����ƽ�⣺

 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������
| A������������Һ�м�CaO����Һ��pH���� |
| B������Һ���ȣ���Һ��pH���� |
| C������Һ�м���Na2CO3��Һ�����й����������� |
| D������Һ�м�������NaOH���壬Ca��OH��2������������ |
����������ȷ���� (����)
| A���к͵�����������ʵ���Ũ�ȵ�����ʹ�����Һ�����������������ƶ��ڴ��� |
| B��������������Һ�Ͱ�ˮ��ϡ��һ�������ߵ�c(OH��)�����ٵ�ԭ����һ�� |
| C�������£�ij��Һ����ˮ�������c(OH��)��1��10��10mol/L������Һ���������� |
| D�������������ʵ���Ũ���Ǵ�����������������c(H��)Ҳ�Ǵ�������� |
�����£�����0.10 mol��L��1�İ�ˮ�������ж���ȷ���ǣ�������
| A����AlCl3��Һ������Ӧ�����ӷ���ʽΪAl3����3OH��=Al��OH��3�� |
| B����ˮϡ�ͺ���Һ��c��NH4+����c��OH������� |
| C����HNO3��Һ��ȫ�кͺ���Һ�������� |
| D������Һ��pH��13 |
Ϊ̽��ij���ε�ˮ�������ȷ�Ӧ������λͬѧ�ֱ����������ʵ�鷽����
| ͬѧ | ʵ����� |
| �� | ������茶�������ˮ����ˮ���½���˵�������ˮ�������ȵ� |
| �� | ������ʹ��Һ�е�Fe3��ת����Fe(OH)3������˵��Fe3��ˮ�������ȵ� |
| �� | ͨ��ʵ�鷢��ͬŨ�ȵ��ȵĴ�����Һ����Ĵ�����Һȥ����Ч���ã�˵��̼����ˮ�������ȵ� |
| �� | �ڴ�������Һ�е����̪��Һ������(������ˮ����)������ɫ���˵��������ˮ�������ȵ� |
���в���ȷ����(����)
A���� B���� C���� D����
�����£���0.100 mol/L NaOH ��Һ�ֱ�ζ�20.00 mL 0.100 mol/L������ʹ��ᣬ�ζ���������ͼ��ʾ������˵����ȷ���ǣ� ��
| A����ֱ��ʾ����ʹ���ĵζ����� |
B��V(NaOH)��10.00 mL ʱ�� ��1 ��1 |
| C��pH��7ʱ������������NaOH��Һ�������� |
| D��V(NaOH)��20 .00 mL ʱ��c(Cl��)��c(CH3COO��) |