��Ŀ����
[��ѧ��ѡ��ѧ�뼼��] (15��)
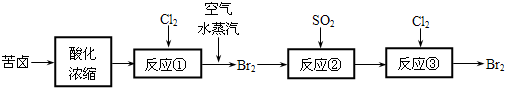
H2O2��ˮ��Һ��һ�ֳ��õ�ɱ������
��1��H2O2�ɵĹ�ҵ�Ʒ�����PtΪ������ʯīΪ����������������Һ���ٽ�������ˮ�⡣��ѧ����ʽΪ��
 ��д��������������Һ��������������Ӧ����
��д��������������Һ��������������Ӧ����
������ ��
������ ��
��2��H2O2�µĹ�ҵ�Ʒ����Ƚ��һ��������ԭ���ٽ��м����ȥ����������H2O2����Ӧ����ʽΪ

�һ������ڴ˱仯�����е������Լ���ɵĹ�ҵ�Ʒ�����¹�ҵ�Ʒ����ŵ���
��
��3��H2O2��ʵ�����Ʒ�֮һ�ǽ������������뵽ϡ�����У��÷�Ӧ�Ļ�ѧ����ʽΪ
��4��д��һ�ֶ����ⶨH2O2ˮ��Һ��H2O2�����Ļ�ѧ����ʽ
��5��д������(4)��Ӧԭ���ļ�Ҫʵ�鲽��
��1������(ʯī)��2H+��2e����H2����2�֣�����(Pt)��2HSO4����2e����S2O82����2H+��2�֣�
��2��������2�֣� �һ�������ѭ��ʹ��(���ȽϾ��õ�)��2�֣�
��3�� BaO2��H2SO4��BaSO4����H2O2��2�֣�
��4��2MnO4����5H2O2��6H����2Mn2����5O2����8H2O ��2�֣������������𰸸��֣�
��5����ȷ��ȡһ������H2O2��Һ��������
���ñ�KMnO4��Һ���еζ������������һ�Σ���Һ��Ϊ��ɫ��ֹͣ�ζ�����ȡ�����
���ظ���2��3�Σ�ȡƽ��ֵ���м��㡣 ��3�֣�
��������

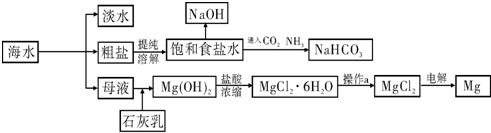
��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��
��ش��������⣺
��1�����о�һ�ֺ�ˮ�����ķ���
��2����ҵ�ϳ������ӽ���Ĥ��������NaOH���������д���ͨ�����ӽ���Ĥ��������
��3�������Ƽ��������ʳ��ˮ��ͨ��CO2��NH3�Ƶ�NaHCO3����ͨ��
���ѧʽ����������
��4��þ��һ����;�ܹ㷺�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ����֪�й����ʵ��۷е��������£�
| MgO | MgCl2 | |
| �۵�/�� | 2852 | 714 |
| �е�/�� | 3600 | 1412 |
 2NH3��+CaCl2+2H2O
2NH3��+CaCl2+2H2O Na2CO3+CO2��+H2O
Na2CO3+CO2��+H2O