��Ŀ����
ijͬѧ̽��SO2�й����ʡ�
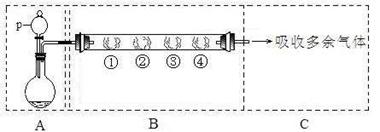
��1����SO2ͨ��BaCl2��Һ�У���������Һ�ֳ�2�ݣ��ڵ�һ���м���NaOH��Һ���ڵڶ����е���FeCl3��Һ�����ݶ��а�ɫ������BaCl2��Һ��ͨ��SO2���������Ϊ ���ڵڶ�����Һ�е���FeCl3��Һʱ��SO2���� �ԣ���Ӧ�����ӷ���ʽΪ �����ɳ����Ļ�ѧʽΪ ��
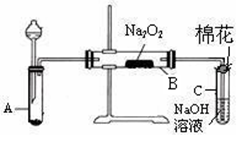
��2���������������SO2������� ��
��3����ҵ������SO2Ϊ7%���������������ͬ��ͬѹ�£������Ϸ����÷����е�O2������SO3����Ӧ���������Ϊԭ�������96.64%����SO2��ת����Ϊ ��
��4����ԭ��33 16S�ĵ����Ų�ʽΪ ��
��1����SO2ͨ��BaCl2��Һ�У���������Һ�ֳ�2�ݣ��ڵ�һ���м���NaOH��Һ���ڵڶ����е���FeCl3��Һ�����ݶ��а�ɫ������BaCl2��Һ��ͨ��SO2���������Ϊ ���ڵڶ�����Һ�е���FeCl3��Һʱ��SO2���� �ԣ���Ӧ�����ӷ���ʽΪ �����ɳ����Ļ�ѧʽΪ ��
��2���������������SO2������� ��
| A��KMnO4(aq) | B��������ˮ | C��ʯ��ˮ | D������ |
��4����ԭ��33 16S�ĵ����Ų�ʽΪ ��
��1������������ ��ԭ�ԣ� 2Fe3����H2SO3��H2O=2Fe2����SO42����4H�� �� BaSO4
��2��D ��3��96% ��4��1s22s22p63s23p4
��2��D ��3��96% ��4��1s22s22p63s23p4
�����������1�����ݸ��ֽⷴӦ��������������������HCl>H2SO3 ��SO2ͨ��BaCl2��Һ�в��������ʷ�����Ӧ��ֻ��ˮ��Ӧ����H2SO3 �ʵõ�BaCl2��H2SO3���Һ���ټ���NaOH��Һʱ������Ӧ2NaOH+H2SO3=Na2SO3+H2O, Na2SO3��BaCl2�������ӷ�Ӧ����BaSO3��ɫ��������һ�ݵ���FeCl3��Һ������������ԭ��Ӧ��2FeCl3+SO2+2H2O=2FeCl2+2HCl+H2SO4 , BaCl2��H2SO4���� BaSO4��ɫ�������ڸ÷�Ӧ��SO2��S���ϼ����ߣ�����Ϊ��ԭ�ԣ���Ӧ�����ӷ���ʽΪ��2Fe3����H2SO3��H2O=2Fe2����SO42����4H�� �����ɳ����Ļ�ѧʽΪBaSO4��
��2��SO2�л�ԭ�ԡ����ԣ�����KMnO4(aq)��ˮ��ʯ��ˮ��Ӧ����������SO2�У�����ܷ�Ӧ���ʲ��������գ�������ȷѡ��ΪD.
��3��������Ӧ��2SO2+O2= 2SO3�������⡣���跴Ӧǰ�����Ϊ100������SO2���Ϊ7������Ӧ�������Ϊ96.64�����ݷ���ʽ�ɿ�������2�����SO2��Ӧ����Ӧ�����������1��������ڼ���100-96.64=3.36����������Ӧ��SO2�����6.72��������SO2��ת����Ϊ6.72L��7L��100%=96%��4��SΪ16��Ԫ�أ������ܼ�ͼ��֪������Ų�ʽΪ1s22s22p63s23p4.

��ϰ��ϵ�д�
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
�����Ŀ
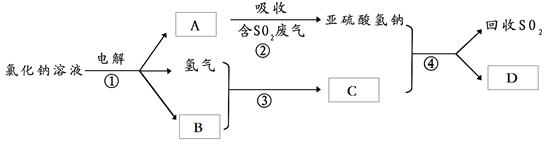 ��ҵ�����У�����۵ķ�Ӧ����Ϊ ��
��ҵ�����У�����۵ķ�Ӧ����Ϊ ��