��Ŀ����
����Ŀ���ֱ�ȡ1 molA��B��C��D��E��F�����л��ʹ���dz��ȼ�գ�������44.8LCO2(���)��D��E��̼���⡢���Ļ��������Ϊͬ���칹�壬E��������A��A����������B��C��F���ܷ����Ӿ۷�Ӧ��C���Ȼ���ӳ�����F�����ƶ��л�������A��B��C��D��E��F�Ľṹ��ʽ��
(1)A��______��B��______��C��______��D��______��E��______��F��______��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ
E��A��_________________________________________��
ʵ�����Ʊ�C��__________________________________��
���𰸡�CH3CHO CH3COOH CH![]() CH CH3OCH3 CH3CH2OH CH2=CHCl 2CH3CH2OH+O2
CH CH3OCH3 CH3CH2OH CH2=CHCl 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CaC2+2H2O��Ca(OH)2+CH��CH��
2CH3CHO+2H2O CaC2+2H2O��Ca(OH)2+CH��CH��
��������
���ȸ���n=![]() ����CO2�����ʵ�����Ȼ�����A��B��C��D��E��F�����л�������к���Cԭ����ĿΪ2���������ʵ����ʼ�ת����ϵ���Ƴ��������ʵĽṹ������д����Ӧ��Ӧ�Ļ�ѧ����ʽ��
����CO2�����ʵ�����Ȼ�����A��B��C��D��E��F�����л�������к���Cԭ����ĿΪ2���������ʵ����ʼ�ת����ϵ���Ƴ��������ʵĽṹ������д����Ӧ��Ӧ�Ļ�ѧ����ʽ��
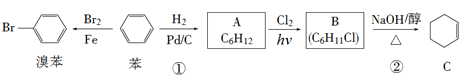
�ڱ�״����44.8LCO2�����ʵ���n(CO2)=![]() =2mol������1 molA��B��C��D��E��F�����л���ȼ�ն�����2molCO2������A��B��C��D��E��F�����л�������к���Cԭ����ĿΪ2mol��1mol=2��E��������A��A����������B����E����-OH��A����-CHO��B����-COOH��E��̼���⡢������Ԫ�صĻ������EΪ�Ҵ���AΪ��ȩ��BΪ���ᣬD��E����Ϊͬ���칹�壬DΪ�����ѣ�C��F�������ۺϷ�Ӧ��C��HCl�ӳ�����F��˵��C��F�����в����ͼ������CΪ��Ȳ��FΪ����ϩ��
=2mol������1 molA��B��C��D��E��F�����л���ȼ�ն�����2molCO2������A��B��C��D��E��F�����л�������к���Cԭ����ĿΪ2mol��1mol=2��E��������A��A����������B����E����-OH��A����-CHO��B����-COOH��E��̼���⡢������Ԫ�صĻ������EΪ�Ҵ���AΪ��ȩ��BΪ���ᣬD��E����Ϊͬ���칹�壬DΪ�����ѣ�C��F�������ۺϷ�Ӧ��C��HCl�ӳ�����F��˵��C��F�����в����ͼ������CΪ��Ȳ��FΪ����ϩ��
(1)��������������֪��A����ȩ��A�ṹ��ʽΪCH3CHO��BΪ���ᣬ�ṹ��ʽΪCH3COOH��CΪ��Ȳ���ṹ��ʽΪCH��CH��DΪ�����ѣ��ṹ��ʽΪCH3OCH3��EΪ�Ҵ����ṹ��ʽΪCH3CH2OH��FΪ����ϩ���ṹ��ʽΪCH2=CHCl��
(2)EΪCH3CH2OH�������ǻ�����O2�ڴ������ڵ������¼��ȣ�����������Ӧ��ȩ���÷�Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��CΪ��ȲCH��CH����ʵ���������õ�ʯCaC2��H2O��Ӧ��ȡ�ģ���Ӧ����ʽΪ��CaC2+2H2O��Ca(OH)2+CH��CH����
2CH3CHO+2H2O��CΪ��ȲCH��CH����ʵ���������õ�ʯCaC2��H2O��Ӧ��ȡ�ģ���Ӧ����ʽΪ��CaC2+2H2O��Ca(OH)2+CH��CH����

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�����Ŀ����������ʵ��������������ó��Ľ�����ȷ����
ѡ�� | ʵ����������� | �� �� |
A | ��һ��Ũ�ȵ�Na2SiO3 ��Һ��ͨ������CO2 ���壬 ���ְ�ɫ������ | H2SiO3 �����Ա�H2CO3������ǿ |
B | ������Fe(NO3)2��ˮ�ܽ�μ�ϡ�����ữ���ٵμ�KSCN��Һ����Һ���Ѫ��ɫ | Fe(NO3)2�ѱ��� |
C | �����£���ã�0.1mol��L-1 Na2SO3��Һ��pHԼΪ10��0.1mol��L-1 NaHSO3��Һ��pHԼΪ5�� | HSO3�� ���H+ ��������SO32����ǿ |
D | �ֱ���25mL��ˮ��25mL��ˮ�е���6��FeCl3 ������Һ��ǰ��Ϊ��ɫ������Ϊ���ɫ�� | �¶����ߣ�Fe3+��ˮ��̶����� |
A.AB.BC.CD.D
����Ŀ�������ˮ��Һ�д��ڵ���ƽ�⡢ˮ��ƽ�⡢�ܽ�ƽ�⣬��ش��������⡣
��. ��֪��������ĵ��볣�����±���
���� | HCOOH | HCN | H2CO3 |
���볣��(25��) | Ka= 1.77��10-4 | Ka=4.3��l0-10 | Ka1=5.0��l0-7Ka2=5.6��l0-11 |
��1��0.1 moI/L NaCN��Һ��0.1mol/L NaHCO3��Һ�У�c��CN����______c��HCO3 �������>������<����=������
��2�������£�pH��ͬ��������ҺA��HCOONa B��NaCN C��Na2CO3�������ʵ���Ũ���ɴ�С��˳����________�����ţ���
��3�������£�����Ũ�ȵ�HCOONa��ҺpH =9�������ӷ���ʽ��ʾ��Һ�ʼ��Ե�ԭ����___________��
��. �����£���0.100 mol/L������Һ�ζ�20.00mL0.l00mol/L ��ij��ˮ��Һ���ζ�������ͼ��ʾ��

��4��d����ʾ����Һ������Ũ���ɴ�С��˳������Ϊ_______________��
��5��b����ʾ����Һ��c(NH3��H2O) �� c(NH4+)=______������Һ�е���������Ũ�ȱ�ʾ����
��. ��֪Ksp(BaCO3) =2.6��l0-9��Ksp( BaSO4)=1.1��10-10.
��6������BaSO4�������Һ�еμ�Na2CO3��Һ������BaCO3��������ʱ����Һ��![]() =___________��������λ��Ч���֣���
=___________��������λ��Ч���֣���