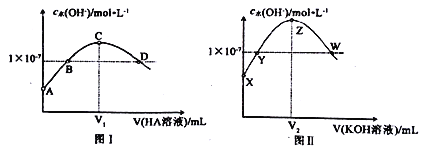
��Ŀ����
����Ŀ����1��.FeCl3����ѧʵ���ҳ��õ��Լ������������Ʊ������������塣
�����Ʊ�������������IJ���������ȷ����____________������ĸ����
A�����Ȼ�����Һ�еμ���������������ϡ��Һ
B����������Ȼ���������Һ
C���ڰ�ˮ�еμ��Ȼ���Ũ��Һ
D���ڷ�ˮ�еμӱ����Ȼ�����Һ�������Һ��ʺ��ɫ��
��2��д��Ba(OH)2��Һ������NaHCO3��Һ��Ӧ�����ӷ���ʽ:___________________��
��3��д��������ĵ���ʽ��________________��д�����������д��ڵ����л�ѧ�����ͣ�____________________��
��4�������к������ۣ��ɼ�______��ȥ���ʣ������Ļ�ѧ����ʽΪ______________��
���𰸡�D Ba2++OH-+HCO3-=BaCO3��+H2O ![]() ���Ӽ����ۼ��������Ӽ��ͷǼ��Լ��� ����������Һ 2Al+2NaOH+2H2O=2NaAlO2+3H2��
���Ӽ����ۼ��������Ӽ��ͷǼ��Լ��� ����������Һ 2Al+2NaOH+2H2O=2NaAlO2+3H2��
��������
(1)ʵ�����Ʊ�������������ķ���Ϊ�����ˮ�еμӱ����Ȼ�����Һ���ȵ����ɫ�������Ǿ�һ�ȶ��ķ�ɢϵ��
(2)��ʽ����Ӧ��Ҫ�Բ�����������Ϊ��д���ӷ���ʽ��
(3)���ԭ�ӽṹ�����ʽ��˳��д����ʽ���жϻ��ϼۣ�
(4)���ö������ʵIJ�ͬ����ӡ�
(1)A�������Ȼ�����Һ�еμ��������������Ʒ�Ӧ�������������������ò��������������壬ѡ��A����
B���Ȼ�������Һ��ˮ�������������������ȴٽ�ˮ�⣬��л���ֺ��ɫ�������õ�������������������ѡ��B����
C���ڰ�ˮ�еμ��Ȼ���Ũ��Һ��Ӧ�������������������ò��������������壬ѡ��C����
D��ʵ�����Ʊ�������������ķ������ڷ�ˮ�еμӱ����Ȼ�����Һ���ȣ��õ����ɫҺ��Ϊ�����������壬��Ӧ�Ļ�ѧ����ʽΪ��FeCl3+3H2O(��)![]() Fe(OH)3(����)+3HCl��ѡ��D��ȷ��
Fe(OH)3(����)+3HCl��ѡ��D��ȷ��
(2) Ba(OH)2��Һ������NaHCO3��Һ��Ӧʱ��NaHCO3����������ȫ��Ӧ�����ӷ���ʽ�ǣ�Ba2++OH-+HCO3-=BaCO3��+H2O��
(3)�����������Oԭ�ӷֱ���H��Clԭ���γ�һ�Թ��õ��Ӷԣ�����ʽΪ![]() ������������������ԭ�Ӽ��γ�һ���Ǽ��Թ��ۼ���ÿ��Oԭ���ٷֱ������γ��γ����Ӽ���
������������������ԭ�Ӽ��γ�һ���Ǽ��Թ��ۼ���ÿ��Oԭ���ٷֱ������γ��γ����Ӽ���
(4)Al��Fe���DZȽϻ��õĽ��������������ᷢ����Ӧ����Al��������ǿ����Һ��Ӧ��Fe���ܷ�Ӧ�����Գ�ȥ�����к������ۿ����������м�������NaOH��Һ��ȥ����Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�