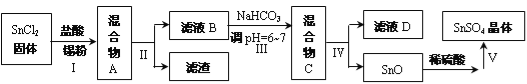
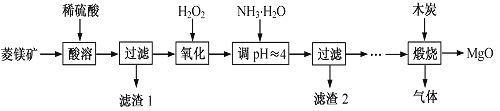
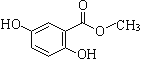
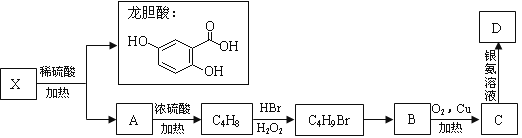
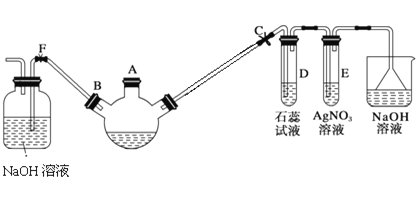
��Ŀ����
����Ŀ����TiO2ת��ΪTiCl4�ǹ�ҵұ�������ѵ���Ҫ��Ӧ֮һ����֪��
TiO2(s)��2Cl2(g)===TiCl4(l)��O2(g) ��H����140.5 kJ��mol��1
C(s��ʯī)��O2(g)===CO(g) ��H����110.5 kJ��mol��1
��ӦTiO2(s)��2Cl2(g)��2C(s��ʯī)===TiCl4(l)��2CO(g)�Ħ�H��
A����80.5 kJ��mol��1 B����30.0 kJ��mol��1
C����30.0 kJ��mol��1 D����80.5 kJ��mol��1
���𰸡�A
��������
�����������֪����TiO2(s)+2Cl2(g)=TiCl4(l)+O2(g) ��H=+140.5kJ/mol����C(s��ʯī)+![]() O2(g)=CO(g)��H=-110.5kJ/mol�����ݸ�˹���ɣ���+�ڡ�2��TiO2(s)+2Cl2(g) +2C(s��ʯī)= =TiCl4(l)+2CO(g)����H=+140.5kJ/mol+(-110.5kJ/mol)��2=-80.5kJ/mol����ѡA��
O2(g)=CO(g)��H=-110.5kJ/mol�����ݸ�˹���ɣ���+�ڡ�2��TiO2(s)+2Cl2(g) +2C(s��ʯī)= =TiCl4(l)+2CO(g)����H=+140.5kJ/mol+(-110.5kJ/mol)��2=-80.5kJ/mol����ѡA��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ