��Ŀ����
11�� �л���A������ʳƷ��ҵ����֪9.0g A������O2�г��ȼ�գ������ɵĻ����������ͨ��������Ũ����ͼ�ʯ�ң��ֱ�����5.4g��13.2g��������ʣ������ΪO2��
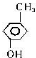
�л���A������ʳƷ��ҵ����֪9.0g A������O2�г��ȼ�գ������ɵĻ����������ͨ��������Ũ����ͼ�ʯ�ң��ֱ�����5.4g��13.2g��������ʣ������ΪO2����1��A���ӵ�����ͼ��ͼ��ʾ����ͼ�п�֪����Է���������90����A�ķ���ʽ��C3H6O3��
��2��A����NaHCO3��Һ������Ӧ��Aһ�����еĹ�����������
�Ȼ���
��3��A���ӵĺ˴Ź���������4���壬�����֮����1��1��1��3����A�Ľṹ��ʽ��CH3CH��OH��COOH��
��4��0.1mol A������Na��Ӧ���ڱ�״���²���H2�������2.24L��
��5��A��һ�������¿ɾۺϵõ�һ�־���������������������ߣ��䷴Ӧ�Ļ�ѧ����ʽ��
 ��
��
���� ��1������Ũ��������5.4gΪˮ����������ʯ������13.2gΪ������̼�����������������غ������Ԫ�ص������������������ʽ���ٽ����Է��������������ʽ��
��2��A����NaHCO3��Һ������Ӧ��Aһ�������Ȼ���-COOH����
��3�����ӵĺ˴Ź���������4���壬˵�������к���4��Hԭ�ӣ������֮����1��1��1��3����4��Hԭ�ӵ���Ŀ֮��Ϊ1��1��1��3����Ϸ���ʽ�жϷ��ӽṹ��
��4������л���Ľṹ�ж����Ƶķ�Ӧ�������������������������
��5�������л���A�Ľṹ��������Ӧԭ����д��Ӧ����ʽ��
��� �⣺��1��5.4gˮ�����ʵ���Ϊ��$\frac{5.4g}{18g/mol}$=0.3mol��n��H��=0.6 mol��13.2g������̼�����ʵ���Ϊ��$\frac{13.2g}{44g/mol}$=0.3mol��n��C��=n��CO2��=0.3 mol�����л���9.0g��OԪ��������9.0g-0.6g-0.3��12 g=4.8 g��n��O��=$\frac{4.8g}{16g/mol}$=0.3 mol����n��C����n��H����n��O��=0.3mol��0.6mol��0.3mol=1��2��1����ʵ��ʽΪCH2O�������ʽΪ��CH2O��n��A����Է�������Ϊ90���ɵ�30n=90����ã�n=3�����л���AΪC3H6O3��
�ʴ�Ϊ��C3H6O3��
��2��A����NaHCO3��Һ������Ӧ��Aһ�������Ȼ���-COOH����
�ʴ�Ϊ���Ȼ���
��3���л���AΪC3H6O3���˴Ź���������4���壬�����֮����1��1��1��3���������4��Hԭ�ӵ���ĿΪ1��1��1��3�������к���1��-COOH��1��-CH3��1�� CH��1��-OH���л���A�Ľṹ��ʽΪCH3CH��OH��COOH��
CH��1��-OH���л���A�Ľṹ��ʽΪCH3CH��OH��COOH��
�ʴ�Ϊ��CH3CH��OH��COOH��
��4���л���A����-OH��-COOH��������Na��Ӧ������������Ӧ�Ļ�ѧ����ʽΪ��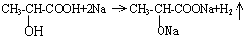 ���ɷ���ʽ��֪0.1molA������Na��Ӧ��������0.1mol���ڱ�״���²���H2������ǣ�0.1mol��22.4L/mol=2.24L��
���ɷ���ʽ��֪0.1molA������Na��Ӧ��������0.1mol���ڱ�״���²���H2������ǣ�0.1mol��22.4L/mol=2.24L��
�ʴ�Ϊ��2.24��
��5���л���A����-OH��-COOH���ۺϷ�Ӧ�õ������ķ�Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л������ʽ��ṹʽ��ȷ���������ŵ����ʵ�֪ʶ����Ŀ�Ѷ��еȣ�ע�����ȼ�շ��������غ�ȷ���л������ʽ�ķ�������ȷ�����л���ṹ�����ʣ�

 һ����������ϵ�д�
һ����������ϵ�д�| ���� | ��� | ������ | |
| A | CH3CH=CH2 | ϩ�� |  |
| B | ��CH3��2 CHCOOH | ���� | -COOH |
| C | HCOOCH2- | ���� | -COOR |
| D | R-OH��R�������� | ���� | -OH |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� |  �������� | B�� |  ������������ | C�� |  ʵ��������ϩ | D�� |  ʯ�͵����� |
| A�� | ʹʯ����Һ����ɫ����Һ | |
| B�� | c��H+��=c��OH-��=10-6mol/L��Һ | |
| C�� | pH=7����Һ | |
| D�� | �����ǡ����ȫ��Ӧ�������ε���Һ |
| A�� | ͨ����� NO���� ������ �� ������ | |
| B�� | ���º����³��� Ne���� ������ �� ������ | |
| C�� | �� ����NO��=2�� �棨N2��ʱ����Ӧ�ﵽƽ�� | |
| D�� | n ��NO����n ��CO����n ��N2����n ��CO2��=2��2��1��2 ʱ����Ӧ�ﵽƽ�� |
| A�� | �ӳɷ�Ӧ | B�� | ��ȥ��Ӧ | C�� | ȡ����Ӧ | D�� | ������Ӧ |
 ��
��