题目内容
乙醇汽油是一种由粮食及各种植物纤维加工成的燃料乙醇和普通汽油按一定比例混配形成的新型替代能源。按照我国的国家标准,乙醇汽油是用90%的普通汽油与10%的乙醇调和而成。
(1)由粮食或各种植物纤维可得到葡萄糖,写出葡萄糖制得乙醇的化学方程式: 。
(2)在常温常压下,1gC2H5OH完全燃烧生成CO2和液态H2O时放出29.71 kJ热量,表示该反应的热化学方程式为 。
(3)下图是一个乙醇燃料电池工作时的示意图,乙池中的两个电极一个是石墨电极,一个是铁电极,工作时M、N两个电极的质量都不减少,请回答下列问题:

①加入乙醇的Pt电极的电极反应式为_________________________。
②在工作过程中,乙池中两电极均收集到标准状况下224mL气体时,甲池中理论上消耗氧气的体积为 mL(标准状况下);若此时乙池溶液体积为200mL,则乙池中溶液的pH为 。
③若要使②中乙池的溶液完全恢复到起始状态,可向乙池中加入 (填代号)
E.0.01molCuSO4
F.0.005molCu2(OH)2CO3
(1)由粮食或各种植物纤维可得到葡萄糖,写出葡萄糖制得乙醇的化学方程式: 。
(2)在常温常压下,1gC2H5OH完全燃烧生成CO2和液态H2O时放出29.71 kJ热量,表示该反应的热化学方程式为 。
(3)下图是一个乙醇燃料电池工作时的示意图,乙池中的两个电极一个是石墨电极,一个是铁电极,工作时M、N两个电极的质量都不减少,请回答下列问题:

①加入乙醇的Pt电极的电极反应式为_________________________。
②在工作过程中,乙池中两电极均收集到标准状况下224mL气体时,甲池中理论上消耗氧气的体积为 mL(标准状况下);若此时乙池溶液体积为200mL,则乙池中溶液的pH为 。
③若要使②中乙池的溶液完全恢复到起始状态,可向乙池中加入 (填代号)
| A.0.01molCu |
| B.0.01molCuO |
| C.0.01molCu(OH)2 |
| D.0.01molCuCO3 |
F.0.005molCu2(OH)2CO3
(1)C6H12O6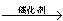 2C2H5OH+2CO2↑(2分)
2C2H5OH+2CO2↑(2分)
(2)C2H5OH(l)+3O2 (g) =2CO2 (g)+3H2O(l)???H="—1366.7" kJ/mol(2分)
(3)①C2H5OH-12e-+16 OH-= 2CO32-+11H2O(2分)
②224(2分);1(2分)
③C(2分,错选不得分)
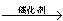 2C2H5OH+2CO2↑(2分)
2C2H5OH+2CO2↑(2分)(2)C2H5OH(l)+3O2 (g) =2CO2 (g)+3H2O(l)???H="—1366.7" kJ/mol(2分)
(3)①C2H5OH-12e-+16 OH-= 2CO32-+11H2O(2分)
②224(2分);1(2分)
③C(2分,错选不得分)
试题分析:(3)②乙池中的两个电极一个是石墨电极,一个是铁电极,工作时M、N两个电极的质量都不减少,表明铁作的是阴极,不参与电极反应,那么在M、N两个电极上发生的电极反应为:
M(阴极):Cu2++2e-=Cu 2H++2e-=H2↑
N(阳极):4OH――4e-=2H2O+O2↑
乙池中两电极均收集到标准状况下224mL气体时,转移的电子物质的量为:0.04mol,那么甲中也转移0.04mol的电子,因此甲池中理论上消耗氧气的体积为224mL。乙池中因为只有0.02mol的氢离子生成氢气,那么溶液中还应该剩余0.02mol的氢离子,那么乙中溶液的氢离子浓度为:c(H+)=0.1mol/L,所以乙池中溶液的pH为1

练习册系列答案
相关题目
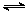 CH3OH(g) △H 1=-90.7 kJ·mol-1
CH3OH(g) △H 1=-90.7 kJ·mol-1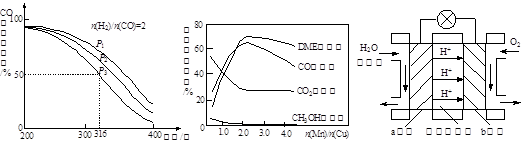


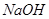 溶液喷淋“捕捉”空气中的
溶液喷淋“捕捉”空气中的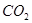 。
。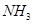 为原料可合成化肥尿素[
为原料可合成化肥尿素[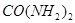 ]。已知:
]。已知:
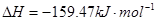 ①
①
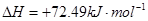 ②
②
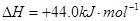 ③
③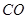 ,在催化剂作用下CO和
,在催化剂作用下CO和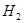 反应生成甲醇:
反应生成甲醇: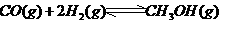 某容积可变的密闭容器中充有10molCO与20mol
某容积可变的密闭容器中充有10molCO与20mol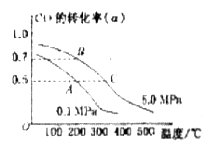
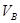 _______VL。(填“大于”、“小于”或“等于”)
_______VL。(填“大于”、“小于”或“等于”)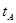 _______
_______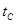 (填“>”、“<”或“=”)
(填“>”、“<”或“=”) cC(气)+dD(气);ΔH=Q,根据图回答:
cC(气)+dD(气);ΔH=Q,根据图回答: