��Ŀ����
��2011?����һģ����1��좣�PH3����һ����ɫ�о綾�����壬�仹ԭ�����Ȱ���NH3��ǿ����һ��ǿ��ԭ����ij��Ӧ��ϵ�д����������ʣ�Cu��H2SO4��CuSO4��PH3��H3PO4��H2O���ش��������⣺
��������Ӧ��ϵ�л�ѧ��Ӧ����ʽΪ
��좣�PH3���ܺ�ˮ�������Ļ�ѧ��Ӧ����ˮ��Һ�������ԣ��÷�Ӧ�������ӷ���ʽ��ʾΪ
��2��������ˮ�к�����ϸС��������ɼ�����ijЩ����ʹ֮�ۼ��ɽϴ�Ŀ����������������һ�ֳ�������ʹ������ˮ��������۳������ʣ��仯ѧʽΪ
��3����CH4����ԭNOX�������������������Ⱦ������
CH4��g��+4NO2��g�� 4NO��g��+CO2 ��g��+2H2O��g����H1=-574kJ?mol-1
CH4��g��+4NO��g�� 2N2��g��+CO2��g��+2H2O��g����H2
��1mol CH4��ԭNO2��N2�����������зų�������Ϊ867kJ�����H2=
��������Ӧ��ϵ�л�ѧ��Ӧ����ʽΪ
PH3+4CuSO4+4H2O=4Cu+H3PO4+4H2SO4
PH3+4CuSO4+4H2O=4Cu+H3PO4+4H2SO4
����좣�PH3���ܺ�ˮ�������Ļ�ѧ��Ӧ����ˮ��Һ�������ԣ��÷�Ӧ�������ӷ���ʽ��ʾΪ
PH3+H2O=PH4++OH-
PH3+H2O=PH4++OH-
����2��������ˮ�к�����ϸС��������ɼ�����ijЩ����ʹ֮�ۼ��ɽϴ�Ŀ����������������һ�ֳ�������ʹ������ˮ��������۳������ʣ��仯ѧʽΪ
KAl��SO4��2.12H2O
KAl��SO4��2.12H2O
����3����CH4����ԭNOX�������������������Ⱦ������
CH4��g��+4NO2��g�� 4NO��g��+CO2 ��g��+2H2O��g����H1=-574kJ?mol-1
CH4��g��+4NO��g�� 2N2��g��+CO2��g��+2H2O��g����H2
��1mol CH4��ԭNO2��N2�����������зų�������Ϊ867kJ�����H2=
-1160kJ/mol
-1160kJ/mol
����������1����PH3����ԭ�����ڷ�Ӧ��ʧ��������H3PO4����CuSO4�ڷ�Ӧ�еõ�������Cu�����ݷ�Ӧ���������д����Ӧ����ʽ��
�ڸ��ݰ�������ˮ�ʼ��Ե�ԭ�����PH3 ��ˮ��Ӧ��Һ�ʼ��Ե�ԭ��
��2����������ˮ��ˮ�����ɵ������������������ԣ��ܾ�ˮ��
��3��CH4��g��+4NO2��g��=4NO��g��+CO2 ��g��+2H2O��g����H1=-574kJ?mol-1��
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2 �ڣ�
������ʽ��+�ڵ�2CH4��g��+4NO2��g��=2N2��g��+2CO2��g��+4H2O����H=��H1+��H2���ݼ���ͷ�Ӧ��֮��Ĺ�ϵʽ�����H2 ��
�ڸ��ݰ�������ˮ�ʼ��Ե�ԭ�����PH3 ��ˮ��Ӧ��Һ�ʼ��Ե�ԭ��
��2����������ˮ��ˮ�����ɵ������������������ԣ��ܾ�ˮ��
��3��CH4��g��+4NO2��g��=4NO��g��+CO2 ��g��+2H2O��g����H1=-574kJ?mol-1��
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2 �ڣ�
������ʽ��+�ڵ�2CH4��g��+4NO2��g��=2N2��g��+2CO2��g��+4H2O����H=��H1+��H2���ݼ���ͷ�Ӧ��֮��Ĺ�ϵʽ�����H2 ��
����⣺��1����PH3����ԭ�����ڷ�Ӧ��ʧ��������H3PO4����CuSO4�ڷ�Ӧ�еõ�������Cu�����Ը÷�Ӧ�ķ���ʽΪPH3+4CuSO4+4H2O=4Cu+H3PO4+4H2SO4��
�ʴ�Ϊ��PH3+4CuSO4+4H2O=4Cu+H3PO4+4H2SO4��
��PH3 ��ˮ������������ӷ�Ӧ����������������Ũ�ȴ���������Ũ�ȣ���Һ�ʼ��ԣ����ӷ���ʽΪ��PH3+H2O=PH4++OH-���ʴ�Ϊ��PH3+H2O=PH4++OH-��
��2����������ˮ��ˮ�����������������壬��������������������ԣ������ܾ�ˮ�������Ļ�ѧʽΪ��KAl��SO4��2.12H2O���ʴ�Ϊ��KAl��SO4��2.12H2O��
��3��CH4��g��+4NO2��g��=4NO��g��+CO2 ��g��+2H2O��g����H1=-574kJ?mol-1��
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2 �ڣ�
������ʽ��+�ڵ�2CH4��g��+4NO2��g��=2N2��g��+2CO2��g��+4H2O����H=��H1+��H2
�����1mol������ȫ��Ӧ�ų�������Ϊx��
2CH4��g��+4NO2��g��=2N2��g��+2CO2��g��+4H2O����H=��H1+��H2
2mol 574kJ+x
1mol 867kJ
x=
-574kJ=1160kJ��
���ԡ�H2=-1160kJ/mol��
�ʴ�Ϊ��-1160kJ/mol��
�ʴ�Ϊ��PH3+4CuSO4+4H2O=4Cu+H3PO4+4H2SO4��
��PH3 ��ˮ������������ӷ�Ӧ����������������Ũ�ȴ���������Ũ�ȣ���Һ�ʼ��ԣ����ӷ���ʽΪ��PH3+H2O=PH4++OH-���ʴ�Ϊ��PH3+H2O=PH4++OH-��
��2����������ˮ��ˮ�����������������壬��������������������ԣ������ܾ�ˮ�������Ļ�ѧʽΪ��KAl��SO4��2.12H2O���ʴ�Ϊ��KAl��SO4��2.12H2O��
��3��CH4��g��+4NO2��g��=4NO��g��+CO2 ��g��+2H2O��g����H1=-574kJ?mol-1��
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2 �ڣ�
������ʽ��+�ڵ�2CH4��g��+4NO2��g��=2N2��g��+2CO2��g��+4H2O����H=��H1+��H2
�����1mol������ȫ��Ӧ�ų�������Ϊx��
2CH4��g��+4NO2��g��=2N2��g��+2CO2��g��+4H2O����H=��H1+��H2
2mol 574kJ+x
1mol 867kJ
x=
| 867kJ��2mol |
| 1mol |
���ԡ�H2=-1160kJ/mol��
�ʴ�Ϊ��-1160kJ/mol��
�����������漰��Ӧ�ȵļ��㡢������ԭ��Ӧ��֪ʶ�㣬��Ӧ�ȵ��йؼ�����ע���˹���ɵ�������ã��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
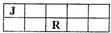 ��2011?����һģ��J��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ�����֪��JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�����˵��������ǣ�������
��2011?����һģ��J��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����ұ�����֪��JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к������Ľ���Ԫ�أ�����˵��������ǣ�������