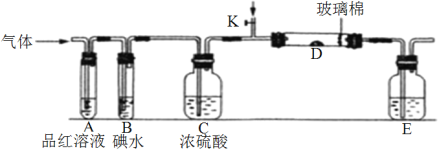
��Ŀ����
����Ŀ��A��B��C��D��E��FΪ����������Ԫ�أ���ԭ��������������A��B ��Ԫ��������ۺ������֮��Ϊ�㡣E��Aͬ���壻D��F�ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء�
(1)д��CԪ�ص�Ԫ������_______���������ڱ��е�λ��__________________��E���ӽṹʾ��ͼΪ__________________��E2D2�ĵ���ʽ________________________��
(2)��A��C��ԭ�Ӹ�����Ϊ5�U1��ɵ�һ�ֻ�������и�ԭ�Ӿ��ﵽ�ȶ��ṹ���û�������������ѧ������Ϊ_________________________��
(3)E2D2��A2D��Ӧ�����ӷ���ʽΪ__________________________________________��
(4)��C��E��ɵ�һ�����ӻ����ﻯѧʽΪE3C���û��������������ӵİ뾶�ɴ�С��˳����____________(��Ԫ�ط��ű�ʾ)���û�������ˮ���ҷ�Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___________________________________��
���𰸡��� ��2���ڵ�VA�� 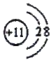
![]() ���Ӽ������ۼ� 2Na2O2+2H2O=4Na++4OH�C+O2�� N3�C>Na+ Na3N+3H2O=3NaOH+NH3��
���Ӽ������ۼ� 2Na2O2+2H2O=4Na++4OH�C+O2�� N3�C>Na+ Na3N+3H2O=3NaOH+NH3��
��������
���������Ϣ������Ԫ�ص����࣬���ݺ�������Ų�����ж�Ԫ�������ڱ��е�λ�ã�����Ԫ�����ʼ����ʵ���ɣ���д���ӽṹʾ��ͼ��������ʵĵ���ʽ���жϻ��ϼ����ͼ���д��ط�Ӧ����ʽ��
A��B��C��D��E��FΪ����������Ԫ�أ���ԭ��������������A��B ��Ԫ��������ۺ������֮��Ϊ�㣬��AΪH��BΪC��E��Aͬ���壬��EΪNa��D��F�ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�أ���DΪO��FΪAl��CΪN��
(1)CԪ��λ��C��O֮�䣬����C��Ԫ������Ϊ�����������ڱ��е�λ��Ϊ��2���ڵ�VA�壻EΪNa�������ӽṹʾ��ͼΪ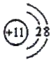 ��E2D2�ǹ������ƣ�����ʽΪ
��E2D2�ǹ������ƣ�����ʽΪ![]() ��
��
�ʴ�Ϊ��������2���ڵ�VA�壻 ��
��![]() ��
��
(2)���������Ϣ֪��A��C��ɵĻ�����Ϊ笠�����(NH4+)���⸺����(H-)��ɵ����ӻ������⻯泥����Ըû�������������ѧ������Ϊ���Ӽ������ۼ���
�ʴ�Ϊ�����Ӽ������ۼ���
(3)���������������������ƺ�ˮ��Ӧ�����ӷ���ʽΪ2Na2O2+2H2O=4Na++4OH�C+O2����
�ʴ�Ϊ��2Na2O2+2H2O=4Na++4OH�C+O2����
(4)CΪ����EΪ�ƣ��γɵļ����Ӻ�������Ų���ͬ���˵��Խ�����Ӱ뾶ԽС�������������ӵİ뾶�ɴ�С��˳����N3�C>Na+���÷�Ӧ�Ļ�ѧ����ʽΪ��Na3N+3H2O=3NaOH+NH3����
�ʴ�Ϊ��N3�C>Na+��Na3N+3H2O=3NaOH+NH3����

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�����Ŀ������������(FeC6H6O7)��һ�������յĸ�Ч���Ƽ��������̷�(FeSO4��7H2O)ͨ�����з�Ӧ�Ʊ���FeSO4+Na2CO3===FeCO3��+Na2SO4 FeCO3 +C6H8O7=FeC6H6O7+CO2��+H2O
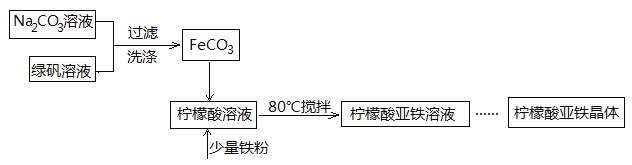
��1�����ɵ�FeCO3�����辭���ϴ�ӣ�����ϴ���Ƿ���ȫ�ķ�����____________________��
��2�����Ƶõ�FeCO3���뵽������������Һ�У��ټ����������ۣ�80���½��跴Ӧ��
�����۵�������_________________��
�ڷ�Ӧ������������ˣ���ȥ�������۵ķ�����_____________��
��3�������Һ��Ũ��������������ˮ�Ҵ������á����ˡ�ϴ�ӡ��������������������塣��������м�����ˮ�Ҵ���Ŀ����________________��
��4��ij�о���ѧϰС����������������(��Ҫ�ɷ�ΪFe2O3��SiO2��Al2O3 ) ���������Ʊ��̷����ٺϳ�������������������ͼ���̷��ܽ�����ߣ����������������������Ʊ�FeSO4��7H2O�����ʵ�鲽��(��ѡ�õ��Լ������ۡ�ϡ�����NaOH��Һ)��
����һ���������м���������ϡ�����ַ�Ӧ������
������Һ�еμӹ�����NaOH��Һ�����ˣ����ϴ�ӹ���
��������м�������ϡ������������ȫ�ܽ�
���ټ������������ۣ���ֽ�����˵õ�FeSO4 ��Һ
��_______________________________________________���õ�FeSO4��7H2O ���塣
��5��ȡ25.00g�������������壨Ħ������Ϊ246g/mol�������100mL��Һ��ȡ20.00mL����ƿ�У���ȡ0.2000mol.L-1������KMnO4����Һװ����ʽ�ζ����У���������ԭ���ⶨ����������������������������ʲ�������KMnO4����Һ��Ӧ����4�εζ���ÿ������KMnO4��Һ��������£�
ʵ����� | 1 | 2 | 3 | 4 |
����KMnO4��Һ��� | 20.00mL | 19.98mL | 21.38mL | 20.02mL |
�ζ��յ�ʱ����Ϊ��__________________________________��������������������________________
����Ŀ���±����и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת������
a | b | c | |
�� | Cu | CuO | Cu��OH��2 |
�� | CH3CH2OH | CH3CHO | CH2=CH2 |
�� | S | SO3 | H2SO4 |
�� | NO | NO2 | HNO3 |
�� | FeCl2 | Fe | FeCl3 |

A. �ڢ� B. �ۢ� C. �ܢ� D. �٢�