��Ŀ����
��ѧ�ܵ�ת������ʵ�����еõ��˹㷺�����á��ش��������⣺
����(1)��25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55 kJ��������д����ʾ����ȼ�յ��Ȼ�ѧ����ʽ�� ��
(2)2Zn��s��+O2��g��=2ZnO��s�� ��H1=" ��702" kJ/mol
2Hg��l��+O2��g��=2HgO��s�� ��H2=" ��182" kJ/mol
�ɴ˿�֪ZnO��s��+Hg��l��= Zn��s��+HgO��s�� ��H3= ��
��3��20����30�����Eyring��Pelzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ������������̬����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��

������ͼΪ������ļ����������أ�
��ش�
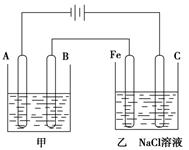
��1���׳���Ϊ�õ��ԭ������ͭ��װ�ã���
A�������������������� ���缫��ӦΪ����������������������
B�������������������� ���缫��ӦΪ����������������������
�������ҺΪ������������������
��2���ҳ���������������̪��Һ����ʼһ��ʱ���Fe������������������ɫ��
��3�����ײ���������12��8g�����Ҳ������ų������ڱ�״���µ����
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ͬʱ���Ҳ�ʣ��Һ��Ϊ400mL�������õ���Һ�����ʵ���Ũ��Ϊ
__ ������ _____��
����(1)��25�桢101kPa�£�1g������ȫȼ������CO2��Һ̬H2O���ų�55 kJ��������д����ʾ����ȼ�յ��Ȼ�ѧ����ʽ�� ��
(2)2Zn��s��+O2��g��=2ZnO��s�� ��H1=" ��702" kJ/mol
2Hg��l��+O2��g��=2HgO��s�� ��H2=" ��182" kJ/mol
�ɴ˿�֪ZnO��s��+Hg��l��= Zn��s��+HgO��s�� ��H3= ��
��3��20����30�����Eyring��Pelzer����ײ���۵Ļ����������ѧ��Ӧ�Ĺ���̬���ۣ���ѧ��Ӧ������ͨ������ײ������ɵģ������ڷ�Ӧ�ﵽ������Ĺ����о���һ������������̬����ͼ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��

������ͼΪ������ļ����������أ�

��ش�
��1���׳���Ϊ�õ��ԭ������ͭ��װ�ã���
A�������������������� ���缫��ӦΪ����������������������
B�������������������� ���缫��ӦΪ����������������������
�������ҺΪ������������������
��2���ҳ���������������̪��Һ����ʼһ��ʱ���Fe������������������ɫ��
��3�����ײ���������12��8g�����Ҳ������ų������ڱ�״���µ����
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��4��ͬʱ���Ҳ�ʣ��Һ��Ϊ400mL�������õ���Һ�����ʵ���Ũ��Ϊ
__ ������ _____��
����1��CH4��g��+2O2��g��==== CO2��g��+ 2H2O��g�� ��H =" ��880" kJ/mol
��2��+260 kJ/mol
��3��NO2��g��+CO��g��="===" CO2��g��+NO��g������H =" ��234" kJ/mol
����1��������ͭ��Cu2++2e-=Cu��������ͭ��Cu-2e-=Cu2+ CuSO4��Һ
��2����
��3��4.48 L
��4��1mol/L
��2��+260 kJ/mol
��3��NO2��g��+CO��g��="===" CO2��g��+NO��g������H =" ��234" kJ/mol
����1��������ͭ��Cu2++2e-=Cu��������ͭ��Cu-2e-=Cu2+ CuSO4��Һ
��2����
��3��4.48 L
��4��1mol/L
����1������Ŀ��Ϣ�������1mol������ȫȼ�������ȶ���������ʱ���ų�������Ϊ16��55=880kJ�������ȼ�յ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g��==== CO2��g��+ 2H2O��g�� ��H =" ��880" kJ/mol
��2�������������Ȼ�ѧ����ʽ�ɵõ���
��ZnO��s��=Zn��s��+1/2O2��g�� ��H1=" +351" kJ/mol
��Hg��l��+1/2O2��g��=HgO��s�� ��H2= ��91kJ/mol
��+�ڵõ���ZnO��s��+Hg��l��= Zn��s��+HgO��s�� ��H3=+260 kJ/mol
��3����ͼ��֪���÷�ӦΪ���ȷ�Ӧ����H3=134��368=��234 kJ/mol�����Ȼ�ѧ����ʽΪ��
NO2��g��+CO��g��="===" CO2��g��+NO��g������H =" ��234" kJ/mol
II��1���׳���Ϊ����ͭ��װ�ã��������ҺӦΪͭ�Σ�����������ͭ��Һ����
��ͭ���������������Դ������������B����Cu-2e-=Cu2+
��ͭ����������A����Cu2++2e-=Cu
��2���ҳ���������Ϊ���ص�������2H2O��2e��=H2����2OH������Һ�ʼ��ԣ������̪��ʺ�ɫ
��3���ײ���������ͭ���أ����Ҳ������ų�������2Cl-2e��=Cl2�����ɹ�ϵʽCu��2e����Cl2������֪�����ڱ�״���µ����Ϊ =4.48L
=4.48L
��4���ɹ�ϵʽ2OH����Cl2����֪��c(OH��)= =1mol/L
=1mol/L
��2�������������Ȼ�ѧ����ʽ�ɵõ���
��ZnO��s��=Zn��s��+1/2O2��g�� ��H1=" +351" kJ/mol
��Hg��l��+1/2O2��g��=HgO��s�� ��H2= ��91kJ/mol
��+�ڵõ���ZnO��s��+Hg��l��= Zn��s��+HgO��s�� ��H3=+260 kJ/mol
��3����ͼ��֪���÷�ӦΪ���ȷ�Ӧ����H3=134��368=��234 kJ/mol�����Ȼ�ѧ����ʽΪ��
NO2��g��+CO��g��="===" CO2��g��+NO��g������H =" ��234" kJ/mol
II��1���׳���Ϊ����ͭ��װ�ã��������ҺӦΪͭ�Σ�����������ͭ��Һ����
��ͭ���������������Դ������������B����Cu-2e-=Cu2+
��ͭ����������A����Cu2++2e-=Cu
��2���ҳ���������Ϊ���ص�������2H2O��2e��=H2����2OH������Һ�ʼ��ԣ������̪��ʺ�ɫ
��3���ײ���������ͭ���أ����Ҳ������ų�������2Cl-2e��=Cl2�����ɹ�ϵʽCu��2e����Cl2������֪�����ڱ�״���µ����Ϊ
 =4.48L
=4.48L��4���ɹ�ϵʽ2OH����Cl2����֪��c(OH��)=
 =1mol/L
=1mol/L
��ϰ��ϵ�д�
�����Ŀ




 ,�÷�Ӧ���ʱ�
,�÷�Ӧ���ʱ� =________kJ/mol (�ú�a��b��ʽ�ӱ�ʾ)��
=________kJ/mol (�ú�a��b��ʽ�ӱ�ʾ)�� �������ڴ����������ṩ���Ӻ͵��ӡ���ͼΪ�õ�ص�ʾ��ͼ����缫d�Ϸ����ĵ缫��ӦʽΪ________________��
�������ڴ����������ṩ���Ӻ͵��ӡ���ͼΪ�õ�ص�ʾ��ͼ����缫d�Ϸ����ĵ缫��ӦʽΪ________________��
 ��ʾ����8molS�γ�8mol S��S����֪ƽ��1molS���е�S��S��Ȼ�����)����Q��_________��
��ʾ����8molS�γ�8mol S��S����֪ƽ��1molS���е�S��S��Ȼ�����)����Q��_________�� 2CH3OH(g) ��H��+37 kJ��mol��1
2CH3OH(g) ��H��+37 kJ��mol��1 ����ͬ��3���ܱ������У�����ͬ��ʽ
����ͬ��3���ܱ������У�����ͬ��ʽ Ͷ�뷴Ӧ����ֺ��¡���ѹ��������Ӧ�٣���÷�Ӧ�ﵽƽ��ʱ���й��������¡�
Ͷ�뷴Ӧ����ֺ��¡���ѹ��������Ӧ�٣���÷�Ӧ�ﵽƽ��ʱ���й��������¡� ��
��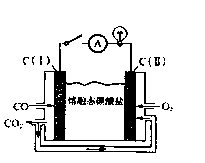
 2Fe3O2(s)+CO2(g) ��H=��47kJ/mol
2Fe3O2(s)+CO2(g) ��H=��47kJ/mol