��Ŀ����
�������л�ѧ����Ҫ���յ������ֱ�Ϊ��H-H:436 kJ/mol��Br-Br:200kJ/mol��H-Br:369kJ/mol������˵����ȷ����(����)
| A��H2��Br2��Ӧ���Ȼ�ѧ����ʽΪ��H2(g)��Br2(g)===2HBr(g)����H����102kJ |
| B��2 L HBr(g)�ֽ��1 L H2(g)��1 L Br2(g)����102 kJ������ |
| C��1 mol H2(g)��1 mol Br2(l)��Ӧ����2 mol HBr(g)�ų�����������102 kJ |
| D������ͬ�����£�1 mol H2(g)��1 mol Br2(g)�������ܺʹ���2 mol HBr(g)������ |
D
A �� �˷�Ӧ��H����102kJ/mol
B �� 2 molHBr(g)�ֽ��1 LmolH2(g)��1 mol Br2(g)����102 kJ������
C ��1 mol H2(g)��1 mol Br2(l)��Ӧ����2 mol HBr(g)�ų�����������102 kJ
D �Դ˷�Ӧ�Ƿ��ȷ�Ӧ����Ӧ��������������������������
B �� 2 molHBr(g)�ֽ��1 LmolH2(g)��1 mol Br2(g)����102 kJ������
C ��1 mol H2(g)��1 mol Br2(l)��Ӧ����2 mol HBr(g)�ų�����������102 kJ
D �Դ˷�Ӧ�Ƿ��ȷ�Ӧ����Ӧ��������������������������

��ϰ��ϵ�д�
�����Ŀ

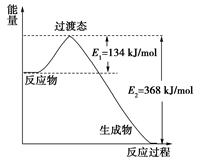
 Z��g��+W��s����H>0�����º��������´ﵽƽ������X��
Z��g��+W��s����H>0�����º��������´ﵽƽ������X�� �ų�46.2 kJ����������N��H���ļ�����_______________________��
�ų�46.2 kJ����������N��H���ļ�����_______________________�� .5 kJ/mol ��
.5 kJ/mol �� (g) ��H�� �D 393.5kJ/mol
(g) ��H�� �D 393.5kJ/mol