��Ŀ����
��14�֣����������е�β������ɻ�����Ⱦ������������տ�������ȾԴ�����������Դ����֪��

��3Fe2O2(s)+CO(g) 2Fe3O2(s)+CO2(g) ��H=��47kJ/mol
2Fe3O2(s)+CO2(g) ��H=��47kJ/mol
��Fe3O3(s)+3CO(g) 2Fe(s)+3CO2(g) ��H=��25kJ/mol
2Fe(s)+3CO2(g) ��H=��25kJ/mol
��Fe3O\4(s)+CO(g) 3FeO(s)+CO2(g) ��H=+19kJ/mol
3FeO(s)+CO2(g) ��H=+19kJ/mol
��1���Լ��㷴Ӧ��FeO��s��+CO��g�� Fe��s��+CO2��g���ġ�H= ����֪1092��÷�Ӧ��ƽ�ⳣ��Ϊ0.357����1200��ʱ�÷�Ӧ��ƽ�ⳣ�� 0.357���>����=����<��������1L���ܱ������У�Ͷ��7.2gFeO��0.1molCO2���ȵ�1092�沢���ָ��¶ȣ���Ӧ��ƽ���������CO������ռ���������Ϊ ��
Fe��s��+CO2��g���ġ�H= ����֪1092��÷�Ӧ��ƽ�ⳣ��Ϊ0.357����1200��ʱ�÷�Ӧ��ƽ�ⳣ�� 0.357���>����=����<��������1L���ܱ������У�Ͷ��7.2gFeO��0.1molCO2���ȵ�1092�沢���ָ��¶ȣ���Ӧ��ƽ���������CO������ռ���������Ϊ ��
��2������β��������ֱ��������̼����ȼ�ϵ�أ�����ԭ������ͼ����ȼ�ϣ����ĵ缫��ӦΪ ��
��3��ת¯���֣�β����CO���������58%��70%��ij�ֳ�����NaOH����CO���ɼ����ƣ�������SO2���ɱ��շۣ�Na2S2O3������д�������ƺ��������ƻ����Һ��SO2���ɱ��շ�ͬʱ���ɶ�����̼�Ļ�ѧ����ʽ ��
��4����550��650��ʱ��β���̳��е�Fe2O3��CO��H2��������ںϳ�����ԭ��Fe3C���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��Ŀǰ�ҹ��������ҵ�ǽ�COת��ΪH2��Ȼ����H2��N2��Ӧ�ϳɵ������ռ���3360m2β��������CO�������Ϊ60%������ѭ���������ٶ�����ת���ʾ�Ϊ100%�������Ͽɻ��NH3 1��

��3Fe2O2(s)+CO(g)
 2Fe3O2(s)+CO2(g) ��H=��47kJ/mol
2Fe3O2(s)+CO2(g) ��H=��47kJ/mol��Fe3O3(s)+3CO(g)
 2Fe(s)+3CO2(g) ��H=��25kJ/mol
2Fe(s)+3CO2(g) ��H=��25kJ/mol��Fe3O\4(s)+CO(g)
 3FeO(s)+CO2(g) ��H=+19kJ/mol
3FeO(s)+CO2(g) ��H=+19kJ/mol��1���Լ��㷴Ӧ��FeO��s��+CO��g��
 Fe��s��+CO2��g���ġ�H= ����֪1092��÷�Ӧ��ƽ�ⳣ��Ϊ0.357����1200��ʱ�÷�Ӧ��ƽ�ⳣ�� 0.357���>����=����<��������1L���ܱ������У�Ͷ��7.2gFeO��0.1molCO2���ȵ�1092�沢���ָ��¶ȣ���Ӧ��ƽ���������CO������ռ���������Ϊ ��
Fe��s��+CO2��g���ġ�H= ����֪1092��÷�Ӧ��ƽ�ⳣ��Ϊ0.357����1200��ʱ�÷�Ӧ��ƽ�ⳣ�� 0.357���>����=����<��������1L���ܱ������У�Ͷ��7.2gFeO��0.1molCO2���ȵ�1092�沢���ָ��¶ȣ���Ӧ��ƽ���������CO������ռ���������Ϊ ����2������β��������ֱ��������̼����ȼ�ϵ�أ�����ԭ������ͼ����ȼ�ϣ����ĵ缫��ӦΪ ��
��3��ת¯���֣�β����CO���������58%��70%��ij�ֳ�����NaOH����CO���ɼ����ƣ�������SO2���ɱ��շۣ�Na2S2O3������д�������ƺ��������ƻ����Һ��SO2���ɱ��շ�ͬʱ���ɶ�����̼�Ļ�ѧ����ʽ ��
��4����550��650��ʱ��β���̳��е�Fe2O3��CO��H2��������ںϳ�����ԭ��Fe3C���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��Ŀǰ�ҹ��������ҵ�ǽ�COת��ΪH2��Ȼ����H2��N2��Ӧ�ϳɵ������ռ���3360m2β��������CO�������Ϊ60%������ѭ���������ٶ�����ת���ʾ�Ϊ100%�������Ͽɻ��NH3 1��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
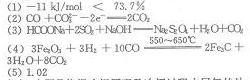

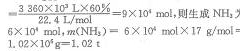

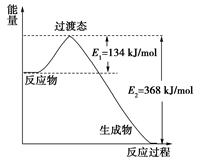
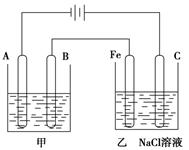

 ��Ӧ��Ӧ�ȵIJⶨ����ش��������⣺
��Ӧ��Ӧ�ȵIJⶨ����ش��������⣺