��Ŀ����
ij������ˮ��Һ��ֻ���ܺ������������е������֣�Na+��NH4+��Ba2+��Cl-��CO32-��SO42-����ȡ����200 mL��Һ��������ʵ�飺�ٵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������1.36 g;�ڵڶ��ݼ�����BaCl2��Һ�ø������12.54 g,����������ϴ�ӡ������������Ϊ4.66 g����������ʵ����ʵ�������Ʋ�����ȷ����( )
| A��һ��������Ba2+�����ܴ���NH4+ |
| B�������ܴ���Ba2+��Cl- |
| C��Na+һ�����ڣ���c(Na+)��0.2 mol/L |
| D��Na+��Cl-���ܴ��� |
C
����

��ϰ��ϵ�д�
�����Ŀ
�������ӷ���ʽ����ȷ����
| A������������Һ���չ����Ķ�����̼ OH����CO2�� HCO3�� |
| B�������������Һ����������������Һ��Ӧ HSO3���� OH���� SO32���� H2O |
| C������ͨ����ˮ�� Cl2��H2O �� Cl�� ��ClO�� ��2H+ |
| D��̼��þ����Һ�мӴ��� CO32����2CH3COOH �� 2CH3COO����CO2����H2O |
���л�����������������ʵ���
| A��BaSO4 | B��HCl | C��CO2 | D��H2O |
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����
| A��������KMnO4��Һ��ͨ��SO2��3SO2+2MnO4-+4OH��=2MnO2��+3SO42-+2H2O |
| B��������Һ��ˮ���е�CaCO3��Ӧ��CaCO3+2H+=Ca2++CO2��+H2O |
| C����FeBr2��Һ��ͨ������Cl2��2Fe2++Cl2=2Fe3++2Cl�� |
| D��������Cu(OH)2�м�����ȩ��Һ�����ȣ� |
 CH3COO��+Cu2O��+3H2O
CH3COO��+Cu2O��+3H2O ����ȷ��ʾ���з�Ӧ�����ӷ���ʽ��(����)
| A���ù�����ˮ���չ�ҵβ���е�SO2��2NH3��H2O��SO2=2NH4+��SO32����H2O |
| B���Ȼ�����Ũ�����ϼ��ȣ�H2SO4��2Cl����,SO2����Cl2����H2O |
| C����������������ϡ���3Fe2����4H����NO3��=3Fe3����NO����3H2O |
| D��������Һ�е���Ba(OH)2��ҺʹSO42��ǡ����ȫ������2Ba2����3OH����Al3����2SO42��=2BaSO4����Al(OH)3�� |
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ���� (����)��
A��̼���Ƶ�ˮ�⣺CO32����2H2O H2CO3��2OH�� H2CO3��2OH�� |
| B����������Һ�еμӹ�����ˮ��Ag����NH3��H2O=AgOH����NH4+ |
C���ö��Ե缫����Ȼ�þ��Һ��2Cl����2H�� H2����Cl2�� H2����Cl2�� |
| D���ù���������ữ�ĺ����ҽ���Һ����ȡ�⣺2I����H2O2��2H��=I2��2H2O |
����A��D���飬ÿ����������Ӧ������������Ӧ����ͬһ�����ӷ���ʽ��ʾ���ǣ� ��
| | ���� | ���� |
| A | ����SO2ͨ��Ba��OH��2��Һ�� | ����SO2ͨ������Ba��OH��2��Һ�� |
| B | ����Ũ��ˮ����Al2��SO4��3��Һ�� | ����Al2��SO4��3��Һ����Ũ��ˮ�� |
| C | 0.1 mol Cl2ͨ�뺬0.2 mol FeBr2����Һ�� | 0.3 mol Cl2ͨ�뺬0.2 mol FeBr2����Һ�� |
| D | ����BaCl2��Һ������Na2SO4��Һ���� | ����Ba��OH��2��Һ�����MgSO4��Һ���� |
��֪���Ը��������Һ���Խ�FeSO4����������ʽΪ2KMnO4+10FeSO4+ 8H2SO4=K2SO4+2MnSO4+5Fe2(SO4)3+8H2O���ֽ�һ�����������ữ�ĸ��������Һ������������Һ��ϣ���ַ�Ӧ������������Һ�м���KI��Һ�������Һ�������ӵ����ʵ���������KI�����ʵ����ı仯��ϵ����ͼ��ʾ��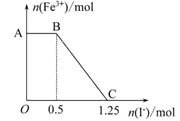
�������й�˵������ȷ����( )
| A��ͼ��AB����Ҫ�Ǹ�����غ͵⻯����Һ��Ӧ |
| B��ͼ��BC�η����ķ�ӦΪ2Fe3++2I-=2Fe2++I2 |
| C������OC�ε����ݿ�֪��ʼ����ĸ�����ص����ʵ���Ϊ0.25 mol |
| D����C���Ժ����Һ�м�������KSCN��Һ����Һ���ɫ |