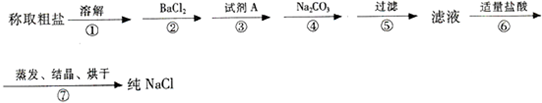
��Ŀ����
��ͼ��II�����dz��������巢��װ�ã�IV��V��VI�������ռ�װ�ã�����Ҫ�������������⣺
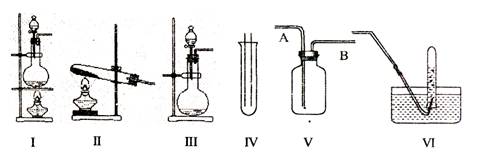
��1�����巢��װ�õ�ѡ������CaCO3�����ᷴӦ��CO2,���ѡ�� ��������ţ�
����NH4Cl��Ca(OH)2��Ӧ��NH3�����ѡ�� ��������ţ�
����MnO2��Ũ���ᷴӦ��ȡ������Ӧѡ��װ�� ��������ţ������ӷ���ʽ��ʾ��ȡ�����ķ�Ӧԭ���� ��
��2���ռ�װ�õ�ѡ����ѡ��VΪ�����ռ�װ�ã�������Ӧ�� ��ͨ�롣�û�ѧ�������������ռ����ķ����� ��
��3����������գ��������鷢�֣���1�����ˮ������336�����HCl���ҵõ�����Һ���ܶ�Ϊ1.08g/cm3,��������Һ�����ʵ����ʵ���Ũ��Ϊ mol/L��

��1�����巢��װ�õ�ѡ������CaCO3�����ᷴӦ��CO2,���ѡ�� ��������ţ�
����NH4Cl��Ca(OH)2��Ӧ��NH3�����ѡ�� ��������ţ�
����MnO2��Ũ���ᷴӦ��ȡ������Ӧѡ��װ�� ��������ţ������ӷ���ʽ��ʾ��ȡ�����ķ�Ӧԭ���� ��
��2���ռ�װ�õ�ѡ����ѡ��VΪ�����ռ�װ�ã�������Ӧ�� ��ͨ�롣�û�ѧ�������������ռ����ķ����� ��
��3����������գ��������鷢�֣���1�����ˮ������336�����HCl���ҵõ�����Һ���ܶ�Ϊ1.08g/cm3,��������Һ�����ʵ����ʵ���Ũ��Ϊ mol/L��
��1��III��II��I��MnO2 + 4H+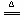 Mn2+ + Cl2 + 2H2O����2��A����ʪ��ĵ⻯�ص�����ֽ����B�ڣ���ֽ��Ϊ��ɫ,֤�������Ѽ�������3��10.5��
Mn2+ + Cl2 + 2H2O����2��A����ʪ��ĵ⻯�ص�����ֽ����B�ڣ���ֽ��Ϊ��ɫ,֤�������Ѽ�������3��10.5��
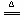 Mn2+ + Cl2 + 2H2O����2��A����ʪ��ĵ⻯�ص�����ֽ����B�ڣ���ֽ��Ϊ��ɫ,֤�������Ѽ�������3��10.5��
Mn2+ + Cl2 + 2H2O����2��A����ʪ��ĵ⻯�ص�����ֽ����B�ڣ���ֽ��Ϊ��ɫ,֤�������Ѽ�������3��10.5����1������CaCO3�����ᷴӦ��CO2,���ڸ÷�Ӧ���ص�Ϊ��Ӧ�������ҹ��士Һ������壬���ѡ��װ�â�����NH4Cl��Ca(OH)2��Ӧ��NH3�����ڸ÷�Ӧ���ص�Ϊ��Ӧ��Ҫ�����ҹ��士��������壬���ѡ��II������MnO2��Ũ���ᷴӦ��ȡ���������ڸ÷�Ӧ���ص�Ϊ��Ӧ��Ҫ�����ҹ��士Һ������壬Ӧѡ��װ�â�2�������������ܶȱȿ����Ĵ�����װ�â��ռ���������Ӧ��A�ܿڽ������������������ʣ�����ʪ��ĵ⻯�ص�����ֽ����B�ڣ���ֽ��Ϊ��ɫ,֤�������Ѽ�������3�����ڱ�״����1L��ˮ����336L��HCl���壬��������Һ�����ʵ����ʵ���Ũ��Ϊ�� ��10.5 mol/L��
��10.5 mol/L��
 ��10.5 mol/L��
��10.5 mol/L��
��ϰ��ϵ�д�
�����Ŀ
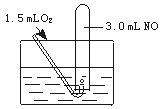
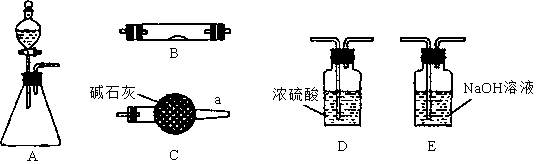
 K2C2O4
K2C2O4