ĢāÄæÄŚČŻ
1£®ÓĆ4ÖÖČÜŅŗ½ųŠŠŹµŃ飬ĻĀ±ķÖŠ”°²Ł×÷¼°ĻÖĻó”±Óė”°ČÜŅŗ”±¶ŌÓ¦¹ŲĻµ“ķĪóµÄŹĒ£Ø””””£©| Ń”Ļī | ²Ł×÷¼°ĻÖĻó | ČÜŅŗ |
| A | ĶØČėCO2£¬ČÜŅŗ²»±ä»ė×Ē£®ĻČĶØČėCO2ŌŁĶØČė°±Ęų£¬ČÜŅŗ±ä»ė×Ē | CaCl2ČÜŅŗ |
| B | ĶØČėCO2£¬ČÜŅŗ±ä»ė×Ē£®¼ĢŠųĶØCO2ÖĮ¹żĮ棬»ė×ĒĻūŹ§£® | Na2SiO3ČÜŅŗ |
| C | ĶØČėCO2£¬ČÜŅŗ±ä»ė×Ē£®ŌŁ¼ÓČėĘ·ŗģČÜŅŗ£¬ŗģÉ«ĶŹČ„£® | Ca£ØClO£©2ČÜŅŗ |
| D | ĶØČėCO2£¬ČÜŅŗ±ä»ė×Ē£®¼ĢŠųĶØCO2ÖĮ¹żĮ棬»ė×ĒĻūŹ§£®ŌŁ¼ÓČė×ćĮæNaOHČÜŅŗ£¬ÓÖ±ä»ė×Ē£® | Ca£ØOH£©2ČÜŅŗ |
| A£® | A | B£® | B | C£® | C | D£® | D |
·ÖĪö A£®ĶØČė°±Ęų£¬Óė¶žŃõ»ÆĢ¼·“Ӧɜ³ÉĢ¼Ėįļ§£¬ÓėĀČ»ÆøĘ·“Ӧɜ³ÉĢ¼ĖįøĘ³Įµķ£»
B£®Na2SiO3ČÜŅŗÓėCO2·“Ӧɜ³É¹čĖį³Įµķ£»
C£®Ca£ØClO£©2ČÜŅŗÓėCO2·“Ӧɜ³ÉHClO£¬HClO¾ßÓŠĘư׊Ō£»
D£®Ca£ØOH£©2ČÜŅŗĶØČėCO2£¬Éś³ÉĢ¼ĖįøĘ³Įµķ£¬¼ĢŠųĶØCO2ÖĮ¹żĮæÉś³ÉĢ¼ĖįĒāøĘ£®
½ā“š ½ā£ŗA£®CaCl2ČÜŅŗ²»ÓėCO2·“Ó¦£¬µ«ĶØČėNH3ŗóČÜŅŗ±äĪŖ£ØNH4£©2CO3æɲśÉś»ė×Ē£¬¹ŹAÕżČ·£»
B£®Na2SiO3ČÜŅŗÓėCO2·“Ӧɜ³É¹čĖį³Įµķ£¬¼ĢŠųĶØCO2ÖĮ¹żĮ棬»ė×Ē²»ĻūŹ§£¬¹ŹB“ķĪó£»
C£®Ca£ØClO£©2ČÜŅŗÓėCO2·“Ӧɜ³ÉHClO£¬HClO¾ßÓŠĘư׊Ō£¬æÉŹ¹Ę·ŗģČÜŅŗĶŹÉ«£¬¹ŹCÕżČ·£»
D£®Ca£ØOH£©2ČÜŅŗĶØČėCO2£¬Éś³ÉĢ¼ĖįøĘ³Įµķ£¬¼ĢŠųĶØCO2ÖĮ¹żĮæÉś³ÉĢ¼ĖįĒāøĘ£¬Ģ¼ĖįĒāøĘÓėNaOH·“Ó¦ÓÖÉś³ÉĢ¼ĖįøĘ³Įµķ£¬¹ŹDÕżČ·£®
¹ŹŃ”B£®
µćĘĄ ±¾Ģāæ¼²é»ÆѧŹµŃé·½°øµÄĘĄ¼Ū£¬ĢāÄæÄŃ¶Č²»“ó£¬×¢Ņā°ŃĪÕĻą¹ŲĪļÖŹµÄŠŌÖŹ£¬ĪŖ½ā“šøĆĢāµÄ¹Ų¼ü£®

Į·Ļ°²įĻµĮŠ“š°ø
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
Ļą¹ŲĢāÄæ
9£®¶ĢÖÜĘŚŌŖĖŲX”¢Y”¢ZµÄŌ×ÓŠņŹżŅĄ“ĪµŻŌö£¬ĘäŌ×ÓµÄ×īĶā²ćµē×ÓŹżÖ®ŗĶĪŖ11£»Y”¢ZŌŚĶ¬Ņ»ÖÜĘŚ£»ZŌ×Ó×īĶā²ćµē×ÓŹżŹĒXŌ×ÓÄŚ²ćµē×ÓŹżµÄ2±¶£¬Ņ²ŹĒYŌ×Ó×īĶā²ćµē×ÓŹżµÄ2±¶£®ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ£Ø””””£©
| A£® | Ąė×Ó°ė¾¶£ŗY£¾X | B£® | ĘųĢ¬Ēā»ÆĪļµÄĪČ¶ØŠŌ£ŗZ£¾X | ||
| C£® | YµÄŃõ»ÆĪļÖŠŗ¬Ąė×Ó¼ü | D£® | ZµÄŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļŹĒĒæĖį |
9£®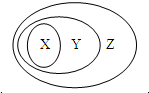 ÓĆĻĀĶ¼±ķŹ¾µÄŅ»Š©ĪļÖŹ»ņøÅÄī¼äµÄ“ÓŹō¹ŲĻµÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©
ÓĆĻĀĶ¼±ķŹ¾µÄŅ»Š©ĪļÖŹ»ņøÅÄī¼äµÄ“ÓŹō¹ŲĻµÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©
 ÓĆĻĀĶ¼±ķŹ¾µÄŅ»Š©ĪļÖŹ»ņøÅÄī¼äµÄ“ÓŹō¹ŲĻµÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©
ÓĆĻĀĶ¼±ķŹ¾µÄŅ»Š©ĪļÖŹ»ņøÅÄī¼äµÄ“ÓŹō¹ŲĻµÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©| X | Y | Z | |
| A | Ńõ»ÆĪļ | »ÆŗĻĪļ | “æ¾»Īļ |
| B | ½ŗĢå | ·ÖÉ¢Ļµ | »ģŗĻĪļ |
| C | µē½āÖŹ | Ėį”¢¼ī”¢ŃĪ | »ÆŗĻĪļ |
| D | ¼īŠŌŃõ»ÆĪļ | ½šŹōŃõ»ÆĪļ | Ńõ»ÆĪļ |
| A£® | A | B£® | B | C£® | C | D£® | D |
6£®ĻĀĮŠ·Ö×ÓÖŠ£¬¼ü½Ē×ī“óµÄŹĒ£Ø””””£©
| A£® | H2S | B£® | H2O | C£® | CCl4 | D£® | NH3 |
13£®ĻĀĮŠ·“Ó¦ÖŠ£¬Įņ±ķĻÖ³öŃõ»ÆŠŌµÄŹĒ£Ø””””£©
| A£® | Ļ”ĮņĖįÓėZnĮ£·“Ó¦ | B£® | SO2ÓėO2·“Ó¦ | ||
| C£® | ÅØĮņĖįÓėĶ·“Ó¦ | D£® | SO3ÓėĖ®·“Ó¦ |
10£®ĻĀĮŠÓŠ¹ŲĢśµÄŠšŹöÕżČ·µÄŹĒ£Ø””””£©
| A£® | ŌŚæÕĘųÖŠ£¬ĢśĖæČēĶ¬Ć¾ĢõŅ»ŃłÄܱ»µćČ¼£¬²¢·¢³öŅ«ŃŪµÄĒæ¹ā | |
| B£® | ĢśŹĒµŲæĒĄļŗ¬Įæ×ī¶ąµÄ½šŹōŌŖĖŲ | |
| C£® | ĢśŌŚøßĪĀĻĀÓėĖ®ÕōĘų·“Ӧɜ³ÉĒāĘųŗĶĖÄŃõ»ÆČżĢś | |
| D£® | ĢśÄÜÓėĻ”ŃĪĖį”¢ÅØĮņĖį”¢Ģ¼ĖįµČĖį·“Ӧɜ³ÉĒāĘųŗĶŃĒĢśŃĪ |
11£®²ā¶ØÉś»īÖŠŅ»Š©ĪļÖŹµÄpH£¬½į¹ūČēĻĀ£ŗ

²ĪÕÕŅŌÉĻĶ¼Ź¾ÅŠ¶Ļ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©

²ĪÕÕŅŌÉĻĶ¼Ź¾ÅŠ¶Ļ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
| A£® | ŃĄøąŹĒÖŠŠŌĪļÖŹ | B£® | ²ŻÄ¾»ŅæÉøÄĮ¼¼īŠŌĶĮČĄ | ||
| C£® | ĪøĖį¹ż¶ąµÄČĖ²»ŅĖ¶ą³ŌéŁ×Ó | D£® | Ź³“×ÄÜŹ¹ĪŽÉ«·ÓĢŖ±äŗģ |
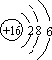 £»»ÆŗĻĪļB2A4µÄµē×ÓŹ½ĪŖ
£»»ÆŗĻĪļB2A4µÄµē×ÓŹ½ĪŖ £®
£® £®
£®