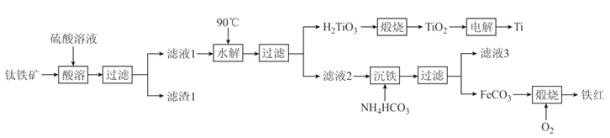
��Ŀ����
����Ŀ���̼��仯�����ڹ�ũҵ�����ͿƼ�������й㷺��Ӧ�á�
������Һ�е�Mn2+�ɱ����ԣ�NH4��2S2O8��Һ����ΪMnO4-���÷��������ڼ���Mn2+��
��1�������ԣ�NH4��2S2O8��Һ����Mn2+ʱ��ʵ������Ϊ________________________��
��2���÷�Ӧ�����ӷ���ʽΪ_____________________��
��3����NH4��2S2O8����Ϊ�������������������ã�������Ľṹ��ʽΪ ����H2S2O8�Ľṹ��ʽΪ ________________��
����H2S2O8�Ľṹ��ʽΪ ________________��
����ʵ�����ú��̷��ϣ���Ҫ�ɷ�ΪMnO2������������Al2O3��MgO��SiO2��Ϊԭ���Ʊ�Mn�Ĺ�����������ͼ��ʾ��

��֪����������������ܶȻ��������±���ʾ��
������ | Fe��OH��3 | Al��OH��3 | Mg��OH��2 | Mn��OH��2 |
�ܶȻ����� | 4.0��10-38 | 1.0��10-33 | 1.8��10-11 | 1.8��10-13 |
������Һ������Ũ����1.0��10-5mol L-1ʱ������Ϊ�����ӳ�����ȫ��
��1���������ʱ��MnO2��Fe����ΪFe3+���÷�Ӧ�����ӷ���ʽΪ________________________���ù����н���ʱ���Һ�̱ȶ��̽����ʵ�Ӱ��ֱ�����ͼ��ʾ��

�����˵Ľ���ʱ���Һ�̱ȷֱ�Ϊ____________��____________��
��2�������������������Һ��c��Mn2+��=0.18molL-1����Ӧ����pH���ķ�ΧΪ________________________��
��3�������ա���Ӧ���������뻹ԭ�������ʵ���֮��Ϊ__________������ԭ��ʱ�������û���Ӧ�ڻ�ѧ���ֽ���__________��
���𰸡���Һ����ɫ��Ϊ�Ϻ�ɫ 5S2O82-+2Mn2++8H2O=2MnO4-+10SO42-+16H+ 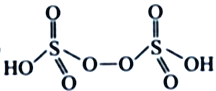 3MnO2+2Fe+12H+=3Mn2++2Fe3++6H2O 60min 3:1
3MnO2+2Fe+12H+=3Mn2++2Fe3++6H2O 60min 3:1 ![]() ��pH��8 1:2 ���ȷ�Ӧ
��pH��8 1:2 ���ȷ�Ӧ
��������
��.�������ȡ�У�ֻ��SiO2�������ᷴӦ����������SiO2�����ݺ�����������м����ΪMnCO3����Fe�����þ���Ϊ�˽��̵Ļ��ϼ۴�+4��ԭ��+2������1�л������˹��������ۡ�����pH���������Ӻ������ӣ�����NaF����þ���ӣ�����̼����麟����̣�����̼���̣��ڿ��������ձ���������ΪMnO2���������ȷ�Ӧ�õ��̵��ʡ�
��.��1����Һ�е�Mn2+ת��ΪMnO4-ʱ��ʵ������Ϊ��Һ����ɫ��Ϊ�Ϻ�ɫ����Ϊ��Һ����ɫ��Ϊ�Ϻ�ɫ��
��2��Mn2+�����ԣ�NH4��2S2O8��Һ����ΪMnO4-����ԭ����ӦΪSO42-����Ӧ�����ӷ���ʽΪ5S2O82-+2Mn2+ +8H2O==2MnO4-+10SO42-+16H+����Ϊ5S2O82-+2Mn2++8H2O=2MnO4-+10SO42-+16H+��
��3��H2S2O8����Ϊ�������������������ã�����Ľṹ��ʽΪ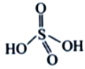 ����H2S2O8�Ľṹ��ʽΪ
����H2S2O8�Ľṹ��ʽΪ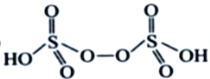 ����Ϊ
����Ϊ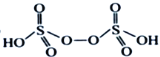 ��
��
��.��1����������Ϣ��֪���������ʱ��MnO2�����Խ����н�Fe����ΪFe3+����������ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ 3MnO2+2Fe+12H+=3Mn2++2Fe3++6H2O����ͼ��֪�����˵Ľ���ʱ��Ϊ60min����ͼ�ҿ�֪�����˵�Һͬ��Ϊ3:1����Ϊ3MnO2+2Fe+12H+=3Mn2++2Fe3++6H2O 60min 3:1��
��2��������ͼ����Ϣ֪������pH����Ŀ����ʹFe3+��A13+������ȫ����Mn2+������������Ksp[A1��OH��3]= 1.0��10-33��,Ksp[Fe��OH��3] = 4.0��10-38��֪��Al3+������ȫʱFe3+�ѳ�����ȫ��Al��OH��3ǡ����ȫ����ʱ��pHΪ-lg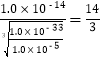 ��Mn2+��ʼ����ʱ��pHΪ-lg
��Mn2+��ʼ����ʱ��pHΪ-lg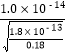 =8��������pH���ķ�ΧΪ
=8��������pH���ķ�ΧΪ![]() ��pH��8����Ϊ
��pH��8����Ϊ![]() ��pH��8��
��pH��8��
��3��������Ϣ֪����������ʱ�������е�O2��MnCO3�� ��ΪMnO2�����ݵ�ʧ�����غ�ɵù�ϵʽO2 2MnCO3�����������뻹ԭ�������ʵ���֮��Ϊ1:2������ԭ��ʱ������������۵����������ķ�Ӧ���ֳ����ȷ�Ӧ����Ϊ1:2 ���ȷ�Ӧ��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�������̿�(��Ҫ�ɷ�ΪMnO2)�����̿�(��Ҫ�ɷ�ΪMnS)Ϊԭ���Ʊ������̾���Ĺ����������£�

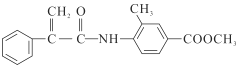
(1)�����д���һ�ַǽ������ʣ�����������еĻ�ѧ����ʽΪ_________________________________��
(2)ʵ���ҳ���������ԭ���ⶨMnSO4��H2O����Ĵ��ȣ�ԭ�����£�2Mn2����NO3-��4PO43-��2H��===2[Mn(PO4)2]3����NO2-��H2O NH4+��NO2-===N2����2H2O [Mn(PO4)2]3����Fe2��===Mn2����[Fe(PO4)2]3������ȡMnSO4��H2O��Ʒ1.000 0 g����������ˮ������������λ���������������泥���220��240 ���³�ַ�Ӧ��Ȼ����N�������ڰ�����������ָʾ������0.100 0 mol��L��1��������隣���Һ�ζ����ɵ�[Mn(PO4)2]3�����յ㡣�ظ�����3�Σ���¼�������±���
�ζ����� | ��Һ����mL | |
�ζ�ǰ | �ζ��� | |
1 | 0.10 | 20.20 |
2 | 1.32 | 21.32 |
3 | 1.05 | 20.95 |
����֪��Fe2����NO2-��2H��===Fe3����NO����H2O��
�ⶨ�����У�����淋�������____________________��____________________��
�����ζ������б���Һ��������鱗��������õģ����ⶨ��MnSO4��H2O����Ĵ��Ƚ���________(����ƫ������ƫ��������������)��
�ۼ�����Ʒ��MnSO4��H2O����������(д���������)__________________��
����Ŀ�����ж������������ݵ���ؽ�����ȷ���ǣ� ��
ѡ�� | ���� | ���� |
A | ��ɰ��֮��ˮ���������ֻ��ɵ�ɰ | ������Ӧ��Ϊ���淴Ӧ |
B | ���������ߣ���ض����࣬��ȡ��ɳճ����Ϊ֮ | �������ߡ�����Ҫ�ɷ�Ϊ������ |
C | ��ʯ��KNO3��������ѩ������ǿ��֮���������� | �����������̡���ԭ��ΪKNO3�ֽ� |
D | �䷨��Ũ�ƾ��Ͳ���ƿ���������ϣ������е�¶ | �����漰�IJ�������Ϊ����Ũ�� |
A. AB. BC. CD. D