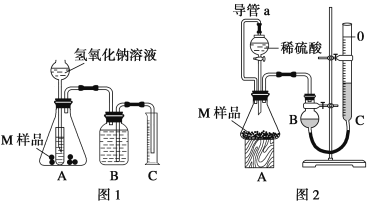
��Ŀ����
����Ŀ���Ҷ�ȩ(OHC-CHO)��ѧ���ʻ��ã��Ƿ�֯��ҵ�г��õ�һ���л�ԭ�ϣ�������������������ά�ķ����Ժͷ����ԡ��乤ҵ����������Ҫ���Ҷ���(HOCH2CH2OH)���������������ȩҺ����������������ش������������:
(1)�Ҷ��������������
���Ҷ���������Ϊԭ�ϣ��ڴ������ڵ������£�250�����ҿ�ʼ�������У������Ҷ�ȩ�������Ҵ���[CH2(OH)COOH]�ķ�Ӧ����ʽ:
I.HOCH2CH2OH(g)+O2(g) ![]() OHC-CHO(g)+2H2O(g)��H1
OHC-CHO(g)+2H2O(g)��H1
II.HOCH2CH2OH(g)+O2(g) ![]() CH2(OH)COOH(g)+H2O(g)��H2
CH2(OH)COOH(g)+H2O(g)��H2
��֪��ӦI����ػ�ѧ��������������:
��ѧ�� | C-H | C-O | H-O | O==O | C==O | C-C |
E/kJ��mol-1 | 413 | 343 | 465 | 498 | 728 | 332 |
�١�H1=_____kJ/mol����ӦI�Ļ�ѧƽ�ⳣ������ʽΪK=________��
�������I��ƽ����ʣ����Բ�ȡ�Ĵ�ʩ��______(����)��
A.�����¶� B.����ѹǿ C.�����¶� D.��Сѹǿ
������Ҷ�ȩ��Ӧѡ���ԵĹؼ�������_________________________��
�ܱ����¶Ⱥ��ݻ����䣬����������˵����ӦI�ﵽƽ��״̬����________(����)��
A.v��(O2)=2v��(H2O)
B.��������ѹǿ������ʱ��仯���仯
C.���������ܶȲ��ٷ����仯
D.�Ҷ�����OHC-CHO�����ʵ���֮��Ϊ1:1
E.����������Ҷ�ȩ������������ٷ����仯
(2)��ȩ(CH3CHO)Һ������������
11.0g40%����ȩ��Һ��40%�����ᣬ��һ������Ͷ��������Ӧ���ڣ���Cu(NO3)2���£������¶���38��40��ʱ����Ӧ10h����ͨ����ȡ�Ȳ�����ȥ��ȩ������ȣ����ѹŨ����4.35g40%�Ҷ�ȩ��Һ��
����ϡ����������ȩ��ȡ�Ҷ�ȩʱ����N2O�������仯ѧ����ʽΪ______________________��
�������������ݣ������Ҷ�ȩ�IJ���Ϊ___________________________��
���𰸡� ��376 ![]() CD ���� BE 2CH3CHO��2HNO3
CD ���� BE 2CH3CHO��2HNO3![]() 2OHC��CHO��N2O����3H2O[Cu��NO3��2д�ɴ���Ҳ��ȷ] 30%
2OHC��CHO��N2O����3H2O[Cu��NO3��2д�ɴ���Ҳ��ȷ] 30%
��������(1)�١�H=��Ӧ��ļ���֮��-������ļ���֮�ͣ���H1=(2��465+4��413+2��343+332+498)kJ/mol -(2��728+332+2��413+4��465)kJ/mol =��376kJ/mol����ӦI�Ļ�ѧƽ�ⳣ������ʽΪK=![]() ���ʴ�Ϊ����376��
���ʴ�Ϊ����376��![]() ��
��
�ڷ�ӦIΪ���ȷ�Ӧ�������I��ƽ����ʣ���Ҫʹƽ�������ƶ���A.�����¶ȣ�ƽ�������ƶ�������B.����ѹǿ��ƽ�������ƶ�������C.�����¶ȣ�ƽ�������ƶ�����ȷ��D.��Сѹǿ��ƽ�������ƶ�����ȷ����ѡCD��
�۴�������ѡ���ԣ�����Ҷ�ȩ��Ӧѡ���ԵĹؼ����������ʺ��ʵĴ������ʴ�Ϊ��������
�ܷ�ӦIΪHOCH2CH2OH(g)+O2(g) ![]() OHC-CHO(g)+2H2O(g)�������¶Ⱥ��ݻ�������A.v��(O2)=2v��(H2O)����ʾ�淴Ӧ����С������Ӧ���ʣ�����ƽ��״̬������B.��Ӧǰ����������ʵ��������仯����������ѹǿ������ʱ��仯���仯���ܹ�˵���ﵽ��ƽ��״̬����ȷ��C.�������������������������䣬���������ܶ�ʼ�ղ��䣬����ƽ��״̬������D.�Ҷ�����OHC-CHO�����ʵ���֮��Ϊ1:1��ƽ��״̬�أ��뷴Ӧ��ʼ������ת�����йأ����ܱ�ʾƽ��״̬������E.����������Ҷ�ȩ������������ٷ����仯��˵����ƽ��״̬����ȷ����ѡBE��
OHC-CHO(g)+2H2O(g)�������¶Ⱥ��ݻ�������A.v��(O2)=2v��(H2O)����ʾ�淴Ӧ����С������Ӧ���ʣ�����ƽ��״̬������B.��Ӧǰ����������ʵ��������仯����������ѹǿ������ʱ��仯���仯���ܹ�˵���ﵽ��ƽ��״̬����ȷ��C.�������������������������䣬���������ܶ�ʼ�ղ��䣬����ƽ��״̬������D.�Ҷ�����OHC-CHO�����ʵ���֮��Ϊ1:1��ƽ��״̬�أ��뷴Ӧ��ʼ������ת�����йأ����ܱ�ʾƽ��״̬������E.����������Ҷ�ȩ������������ٷ����仯��˵����ƽ��״̬����ȷ����ѡBE��
(2)����ϡ����������ȩ��ȡ�Ҷ�ȩʱ����N2O��������Ӧ�Ļ�ѧ����ʽΪ2CH3CHO��2HNO3![]() 2OHC��CHO��N2O����3H2O���ʴ�Ϊ��2CH3CHO��2HNO3
2OHC��CHO��N2O����3H2O���ʴ�Ϊ��2CH3CHO��2HNO3![]() 2OHC��CHO��N2O����3H2O��
2OHC��CHO��N2O����3H2O��
��4.35g40%�Ҷ�ȩ��Һ�к����Ҷ�ȩ�����ʵ���=![]() =0.03mol��11.0g40%����ȩ��Һ�к�����ȩ�����ʵ���=
=0.03mol��11.0g40%����ȩ��Һ�к�����ȩ�����ʵ���=![]() =0.4mol������������0.1mol�Ҷ�ȩ�� �Ҷ�ȩ�IJ���=
=0.4mol������������0.1mol�Ҷ�ȩ�� �Ҷ�ȩ�IJ���=![]() ��100%=30%���ʴ�Ϊ��30%��
��100%=30%���ʴ�Ϊ��30%��

����Ŀ��ij��ѧС����̽�������仯����������Ժͻ�ԭ�ԣ���ش��������⣺
(1)����ͷ�ι��⣬����Ϊ��ʵ��ز���ȱ�ٵ�һ�ֲ���������___________��
(2)��������������ʵ�鱨�棺
ʵ��Ŀ�ģ�̽�������仯����������Ժͻ�ԭ�ԡ�
�Լ������ۡ�FeCl3��Һ��FeCl2��Һ����ˮ��пƬ��ͭƬ��
ʵ���¼(��б�߲��ֲ�����д)��
��� | ʵ������ | ʵ������ | ���ӷ���ʽ | ʵ����� |
�� | ��FeCl2��Һ�е���������ˮ | ��Һ��dz��ɫ��Ϊ�ػ�ɫ | Fe2+���л�ԭ�� | |
�� | ��FeCl2��Һ�м���пƬ |
| Zn��Fe2+��Zn2+��Fe | |
�� | ��FeCl3��Һ�м����������� |
| Fe��2Fe3+��3 Fe2+ | Fe3+���������� |
�� |
|
| Fe3+���������� |
ʵ����ۣ�_________________________________��
(3)�������Ͻ����жϣ����������м��������ԣ����л�ԭ�Ե��У�_____��(�����)
A��Cl2 B��Na C��Na+ D��Cl�� E��SO2 F������