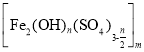��Ŀ����
����Ŀ������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl����Mg2+��Ba2+��CO32����SO42������ȡ���ݸ�100mL����Һ��������ʵ�飺
����һ�ݼ���AgNO3��Һ�г���������
���ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.08mol��
�������ݼ�����BaCl2��Һ�ø������12.54g������������ϴ�ӡ������������Ϊ4.66g����������ʵ�飬�ش��������⣺
��1���ɵڶ��ݽ��е�ʵ���֪�������Ӧ����___________���ӣ������ʵ���Ũ��Ϊ________��
��2���ɵ����ݽ��е�ʵ���֪12.54�˳����ijɷ���______________��д������ѧʽ���������ʵ����ֱ�Ϊ______________________��
��3��ԭ��Һ���Ƿ����K+_______��ǡ�����������K+��Ũ�ȵ�ȡֵ��Χ��____________________���������ڴ˿տɲ��
���𰸡�NH4+ 0.8 mol/L BaCO3��BaSO4 0.04 mol �� 0.02 mol �� �� 0.4 mol/L
��������
�������������һ����Һ����![]() ��Һ�г����������Ƶÿ��ܺ���
��Һ�г����������Ƶÿ��ܺ���![]() ���ڶ�����Һ������
���ڶ�����Һ������![]() ��Һ���Ⱥ��ռ������壬�Ƶ�һ������
��Һ���Ⱥ��ռ������壬�Ƶ�һ������![]() ��һ��������
��һ��������![]() ����������Һ���÷��������ӷ�Ӧ���������㡢�Ƶ�һ������
����������Һ���÷��������ӷ�Ӧ���������㡢�Ƶ�һ������![]() ��һ��������
��һ��������![]() ��������Һ���������ӵĵ���غ㣬�����Ƴ�
��������Һ���������ӵĵ���غ㣬�����Ƴ�![]() һ�����ڡ�
һ�����ڡ�
��1����������![]() ��Һ���Ⱥ��ռ�������0.08mol���壬����Ϊ����������Һ�к���0.08mol
��Һ���Ⱥ��ռ�������0.08mol���壬����Ϊ����������Һ�к���0.08mol![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() ��
��
�ʴ�Ϊ��![]() ��
��![]() ��
��
��2��������![]() ��Һ�ø������12.54g������������ϴ�ӡ������������Ϊ4.66g����֪��������
��Һ�ø������12.54g������������ϴ�ӡ������������Ϊ4.66g����֪��������![]() Ҳ����
Ҳ����![]() ��
��![]() ����Ϊ4.66g�����ʵ���Ϊ��
����Ϊ4.66g�����ʵ���Ϊ��![]() ��̼�ᱵ����Ϊ��
��̼�ᱵ����Ϊ��![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ��
��
�ʴ�Ϊ��![]() ��0.04 mol ��0.02 mol��
��0.04 mol ��0.02 mol��
��3������������������Һ�п϶�����![]() ��
��![]() ���϶�������
���϶�������![]() ��
��![]() �����ܴ���
�����ܴ���![]() ������
������![]() �����ʵ���Ϊ0.04 mol ��0.02 mol��
�����ʵ���Ϊ0.04 mol ��0.02 mol��![]() ���ʵ���Ϊ0.08mol�����ݵ���غ㣬����
���ʵ���Ϊ0.08mol�����ݵ���غ㣬����![]() ��
��![]() �����ʵ���Ϊ0.04mol��������
�����ʵ���Ϊ0.04mol��������![]() ��
��![]() �����ʵ��������0.04mol���ʿ϶�����
�����ʵ��������0.04mol���ʿ϶�����![]() �������ʵ���Ũ�ȴ��ڵ���
�������ʵ���Ũ�ȴ��ڵ���![]() ��
��
�ʴ�Ϊ���� ���� 0.4 mol/L��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����T�������£���1L�̶�������ܱ�����M�м���2molX��1molY���������·�Ӧ��2X(g)��Y(g)![]() a Z(g)��W(g) ��H����890kJ��mol��1(aΪ������)��
a Z(g)��W(g) ��H����890kJ��mol��1(a������)��
����Ӧ�ﵽƽ���Ӧ�ų�������ΪQ1kJ������X��ת����Ϊ������ƽ����������¶ȣ���������ƽ����Է���������С����
��1����ѧ������a��ֵΪ_____��
��2���¶�ά��T �治�䣬����ʼʱ������M�м���4molX��6molY�����ﵽƽ��ʱ�����ڵ�ѹǿ��С��10%����Ӧ�зų�������Ϊ___kJ��
��3���¶�ά��T �治�䣬����һ����ԭ���������ȵĺ�ѹ����N�У�����2molX��1 molY�������Ϸ�Ӧ����ƽ�⣬��������X����������M___N(ѡ�������������)��
��4����֪���÷�Ӧ��ƽ�ⳣ�����¶ȵı仯�����
�¶�/�� | 200 | 250 | 300 | 350 |
ƽ�ⳣ��K | 9.94 | 5.2 | 1 | 0.5 |
����ij�¶��£�2molX��1molY������M�з�Ӧ��ƽ�⣬ X��ƽ��ת����Ϊ50%������¶�Ϊ_____�档ƽ��ʱ X���������Ϊ____��