��Ŀ����
7���������л�ѧ����ʽ����1��2KI+Cl2=2KCl+I2��2��2FeCl2+Cl2=2FeCl3��3��2FeCl3+2HI=2FeCl2+I2+2HCl ��4��I2+SO2+2H2O=H2SO4+2HI
�ж�������������ǿ��˳����ȷ���ǣ�������
| A�� | Cl2��Fe3+��SO2��I2 | B�� | Cl2��Fe3+��I2��SO2 | C�� | Cl2��I2��Fe3+��SO2 | D�� | Fe3+��I2��Cl2��SO2 |
���� ����������ԭ��Ӧ�У��������������Դ���������������жϣ���1��2KI+Cl2=2KCl+I2�У�Cl2������������������ΪI2����2��2FeCl2+Cl2=2FeCl3��Ӧ��Cl2������������������FeCl3����3��2FeCl3+2HI=2FeCl2+2HCl+I2�У�FeCl3������������������ΪI2����4��I2+SO2+2H2O=H2SO4+2HI�У�I2������������������ΪH2SO4��
��� �⣺��1��2KI+Cl2=2KCl+I2�У�Cl2��������������KIΪI2��������Cl2��I2��
��2��2FeCl2+Cl2=2FeCl3��Ӧ��Cl2��������������FeCl2�õ���������FeCl3��������Cl2��FeCl3��
��3��2FeCl3+2HI=2FeCl2+2HCl+I2�У�FeCl3��������������HIΪI2��������FeCl3��I2��
��4��I2+SO2+2H2O=H2SO4+2HI�У�I2��������������SO2ΪH2SO4��������I2��SO2��
���������������ɴ�С��˳��ΪCl2��Fe3+��I2��SO2��
��ѡB��
���� ���⿼����������ԭ��Ӧ��ǿ�����ɷ����жϣ�ע��Ԫ�ػ��ϼ۱仯�ж�������������������ݹ��������������Դ���������������жϵõ�˳����Ŀ�ϼ�

 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�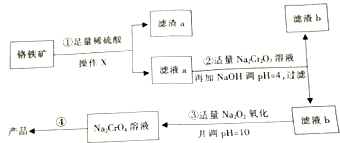 �Ը�������Ҫ�ɷ�FeO•Cr2O3����������SiO2�ȣ�Ϊԭ����ȡ�����ƾ��壨Na2CrO4������������ͼ��ʾ��
�Ը�������Ҫ�ɷ�FeO•Cr2O3����������SiO2�ȣ�Ϊԭ����ȡ�����ƾ��壨Na2CrO4������������ͼ��ʾ����֪����������Һ��Cr��+6���ױ���ԭΪ+3�ۣ���pH��9ʱCr��CrO2-��ʽ�������ױ�������
�ڳ����£�����������������������ʽ����ʱ��Һ��pH���£�
| ������ | Fe3+ | Fe2+ | Cr3+ |
| ��ʼ����ʱ��pH | 2.7 | 7.6 | |
| ������ȫ��pH | 3.7 | 9.6 | 9����9���ܽ� |
��2���ڢܲ��õ���Ʒ�漰���IJ���������Ũ�������½ᾧ�����ˡ�ϴ�ӵȣ�
��3���ڢٲ���Ӧ�����ӷ���ʽ��FeO+2H+=Fe2++H2O��Cr2O3+6H+=2Cr3++3H2O���ڢڲ���������b��ԭ����Fe3+ˮ��������������������
��4����֪��1molAl��ȫȼ�շ���a KJ��1mol Fe��ȫȼ������Fe3O4����b KJ��д��Al��Fe3O4�������ȷ�Ӧ���Ȼ�ѧ����ʽ8Al��s��+3Fe3O4 ��s��=9Fe��s��+4Al2O3 ��s������H=��8a-9b��kJ/mol��
��5��������224kg���������к�FeO•Cr2O3 50%�������������������Եõ�Na2CrO4��Ʒ��������189kg������ÿ����ǡ����ȫת������
| A�� | 0.9 mol H2O | B�� | 0.3 mol H2SO4 | C�� | 0.2 mol NH3 | D�� | 0.4 mol CH4 |
| A�� | 1 | B�� | 2 | C�� | 3 | D�� | 4 |
 Ӫ��ƽ�⡢��ѧʹ��ʳƷ���Ӽ������ڽ����������������
Ӫ��ƽ�⡢��ѧʹ��ʳƷ���Ӽ������ڽ����������������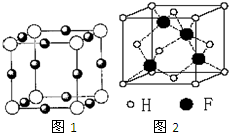 ��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M����ֻ�����ԳɶԵ��ӣ�CԪ���ǵؿ��к�����ߵĽ���Ԫ�أ�D���ʵľ���������ͬ���ڵĵ�����û����ͬ�ģ�Fԭ�Ӻ���������������B��ͬ�����������Ӿ������������������Ϣ���ش��������⣺������ʱ��A��B��C��D��E��F������Ӧ��Ԫ�ط��ű�ʾ��