ЬтФПФкШн
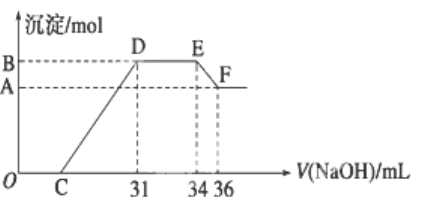
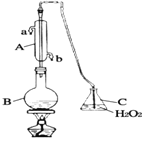
ЁОЬтФПЁПЦЯЬбОЦГЃгУНЙбЧСђЫсФЦЃЈNa2S2O5ЃЉзїПЙбѕЛЏМСЁЃФГаЫШЄаЁзщгУЯТЭМзАжУЃЈМаГжзАжУТдЃЉВтЖЈФГЦЯЬбОЦжаПЙбѕЛЏМСЕФВаСєСПЃЈвдгЮРыЕФSO 2МЦЫуЃЉЃЌЗНАИШчЯТЃКЯђBжаМгШы300.00mLЦЯЬбОЦКЭЪЪСПЕФЯЁСђЫсЃЌМгШШЪЙSO2ШЋВПвнГіВЂгыCжаH2O2ЭъШЋЗДгІЃЌГ§ШЅCжаЙ§СПЕФH2O2КѓЃЌНЋCжавКЬхзЊвЦжСаЁЩеБжаЃЌЯђЩеБФкж№ЕЮМгШыBaCl2ШмвКжСГСЕэСПВЛдйдіМгЃЌЙ§ТЫГіГСЕэЃЌОЯДЕгЁЂИЩдяКѓЃЌГЦЕУЙЬЬхЕФжЪСПЮЊ0.2796gЃЌдђИУЦЯЬбОЦжаSO2ЕФКЌСПЮЊ
A. 0.256 g/L B. 0.04 g/L C. 0.24 g/L D. 0.0768 g/L
ЁОД№АИЁПA
ЁОНтЮіЁП
ЯђBжаМгШы300.00mLЦЯЬбОЦКЭЪЪСПЕФЯЁСђЫсЃЌМгШШЪЙSO2ШЋВПвнГіЃЌвнГіЕФSO2гыCжаH2O2ЭъШЋЗДгІЃЌЫЋбѕЫЎОпгабѕЛЏадЃЌФмЙЛНЋЖўбѕЛЏСђбѕЛЏГЩСђЫсЃЌЗДгІЗНГЬЪНЮЊЃКSO2+H2O2=H2SO4ЃЌГ§ШЅCжаЙ§СПЕФH2O2КѓЃЌНЋCжавКЬхзЊвЦжСаЁЩеБжаЃЌЯђЩеБФкж№ЕЮМгШыBaCl2ШмвКжСГСЕэСПВЛдйдіМгЃЌЗЂЩњЗДгІЕФЗНГЬЪНЮЊЃКH2SO4+BaCl2=BaSO4Ё§+ 2HClЃЌЙ§ТЫГіГСЕэЃЌОЯДЕгЁЂИЩдяКѓЃЌГЦЕУЙЬЬхЕФжЪСПЮЊ0.2796gЃЌДЫЙЬЬхЮЊBaSO4ЁЃИљОнаХЯЂНјааМЦЫуЃК
SO2ЁЊH2SO4ЁЊBaSO4Ё§
64 233
m(SO2 ) 0.2796g
m(SO2 )=0.0768g
дђИУЦЯЬбОЦжаSO2ЕФКЌСПЮЊ=0.0768gЁТ0.3L=0.256 g/LЃЌД№АИбЁAЁЃ