��Ŀ����
17�� ��NH4��2SO4•MnSO4•nH2O��������泥���dz��ɫ���壬������ˮ����ũҵ��������Ԫ�ط��ϣ�ʵ������MnO2�Ʊ�������淋�ʵ�鲽�����£�
��NH4��2SO4•MnSO4•nH2O��������泥���dz��ɫ���壬������ˮ����ũҵ��������Ԫ�ط��ϣ�ʵ������MnO2�Ʊ�������淋�ʵ�鲽�����£�����1�����ձ��м���20.0mL 1mol•L-1���Ტ����2.5g�����ȣ��������ִμ���2.5g MnO2�����ϱ�����ʹ���ַ�Ӧ��
����2�������Һ�����ȹ��ˣ�
����3��������Һ�м���3.0g����泥��������ȫ���ܽ���ڱ�ˮԡ����ȴ��30���Ӻ���ˣ����������Ҵ���Һϴ�����Σ�����ֽ���ɻ���ڱ������ϸ��
����4���Ƶò�Ʒ����Ϊ7.5g��
��1������100mL 1mol•L-1������Һʱ����Ҫ�IJ����������ձ����������⣬����Ҫ100mL����ƿ����ͷ�ιܣ�
��2��MnO2������������Һ��Ӧ�Ļ�ѧ����ʽΪMnO2+H2C2O4+H2SO4=MnSO4+2CO2��+2H2O��
��3�����Ҵ�ϴ�ӵ�Ŀ���Ǽ��ٲ�Ʒ��ʧ��ͬʱ���ڸ��
��4��Ϊ�����Ʒ�к�Mn2+��ȡ������Ʒ����ˮ������NaBiO3���壨�ܣ���ϡ�����ȣ���Һ����ɫ��ͬʱ������ɫ��Bi3+����÷�Ӧ�����ӷ���ʽΪ2Mn2++5NaBiO3+14H+=2MnO4-+5Bi3++5Na++7H2O��
��5����֪�����£�Mn��OH��2��KspΪ2��10-13����0.2mol•L-1�����������Һ�У���Ũ��ˮ����pH��8ʱ��Һ��ʼ���ֳ�������������Һ����仯��
��6��Ϊȷ���仯ѧʽ���ֶԲ�Ʒ�ڵ����н������ط�������֪��371���½ᾧˮ��ȫ��ʧȥ����TG������ͼ��ʾ����TG=������Ʒ��ʣ������/������Ʒ����ʼ������100%����NH4��2SO4•MnSO4•nH2O�е�n=7�������֣���
���� ��1������������Һ�Ļ���������֪������100mL 1mol•L-1������Һʱ����Ҫ�IJ�������Ϊ�ձ�����������100 mL����ƿ����ͷ�ιܣ�
��2��MnO2�������������Һ��ԭΪ�����ӣ����ݵ��ӵ�ʧ�غ��Ԫ���غ���д��Ӧ�Ļ�ѧ����ʽ��
��3���Ҵ��ӷ���������������Ҵ��е��ܽ��С��
��4��Mn2+��NaBiO3���塢ϡ�����ȣ���Ӧ����Һ����ɫ��˵����MnO4-������ͬʱ������ɫ��Bi3+�����ݵ���غ���д��Ӧ�����ӷ���ʽ��
��5������Ksp=c��Mn2+��•c2��OH-��������Һ��c��OH-������������pHֵ��
��6������$\frac{M[��N{H}_{4}��{\;}_{2}SO{\;}_{4}•MnSO{\;}_{4}]}{M[��N{H}_{4}��_{2}S{O}_{4}•MnS{O}_{4}•n{H}_{2}O]}$��100%=1-30.8%�ɼ���n��ֵ��
��� �⣺��1������������Һ�Ļ���������֪������100mL 1mol•L-1������Һʱ����Ҫ�IJ�������Ϊ�ձ�����������100 mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��100 mL����ƿ����ͷ�ιܣ�
��2��MnO2�������������Һ��ԭΪ�����ӣ����ݵ��ӵ�ʧ�غ��Ԫ���غ��֪��Ӧ�Ļ�ѧ����ʽΪMnO2+H2C2O4+H2SO4=MnSO4+2CO2��+2H2O��
�ʴ�Ϊ��MnO2+H2C2O4+H2SO4=MnSO4+2CO2��+2H2O��
��3���Ҵ��ӷ���������������Ҵ��е��ܽ��С���������Ҵ�ϴ�ӵ�Ŀ���Ǽ��ٲ�Ʒ��ʧ��ͬʱ���ڸ��
�ʴ�Ϊ�����ٲ�Ʒ��ʧ��ͬʱ���ڸ��
��4��Mn2+��NaBiO3���塢ϡ�����ȣ���Ӧ����Һ����ɫ��˵����MnO4-������ͬʱ������ɫ��Bi3+����Ӧ�����ӷ���ʽΪ2Mn2++5NaBiO3+14H+=2MnO4-+5Bi3++5Na++7H2O��
�ʴ�Ϊ��2Mn2++5NaBiO3+14H+=2MnO4-+5Bi3++5Na++7H2O��
��5������Ksp=c��Mn2+��•c2��OH-����֪����Һ��c��OH-��=$\sqrt{\frac{K{\;}_{sp}}{c��Mn{\;}^{2+}��}}$=$\sqrt{\frac{2��1{0}^{-13}}{0.2}}$mol•L-1=1��10-6 mol•L-1��������Һ��pH=8��
�ʴ�Ϊ��8��
��6������$\frac{M[��N{H}_{4}��{\;}_{2}SO{\;}_{4}•MnSO{\;}_{4}]}{M[��N{H}_{4}��_{2}S{O}_{4}•MnS{O}_{4}•n{H}_{2}O]}$��100%=1-30.8%�ɵ�$\frac{283}{283+18x}$=1-30.8%������n=7��
�ʴ�Ϊ��7��
���� ���⿼����������茶������ȡ���漰��֪ʶ��Ƚ϶࣬���Ը�����ѧ֪ʶ�����������ó���ȷ���ۣ������Ѷ��еȣ�

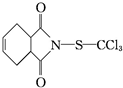 ����һ�ֹ����Ե���Ҫ����Ҷ�汣����ɱ��������ҩ�����ڹ������߲˼����־���������ʹ�ã�����˵����ȷ���ǣ�������
����һ�ֹ����Ե���Ҫ����Ҷ�汣����ɱ��������ҩ�����ڹ������߲˼����־���������ʹ�ã�����˵����ȷ���ǣ�������| A�� | �˾����ķ���ʽΪC9H8O2NSCl3�����ڷ����廯���� | |
| B�� | �˾���������ˮ���ڼ��������²�����NaOH��Һ��Ӧ | |
| C�� | �˾����������CCl4��Һ����ȡ����Ӧʹ֮��ɫ | |
| D�� | �˾�����һ���������ܷ���ȡ�����Ӿ۵ȷ�Ӧ |
| A�� | SO2�л���CO2��ͨ�뱥��NaHSO3��Һ����CO2 | |
| B�� | NaCl��Һ�л���NaAlO2��ͨ������CO2�Խ�AlԪ��ת��ΪAl��OH��3���������˼��� | |
| C�� | ���Ʊ�FeCl2���壬�ɽ�����������������������ȼ�յķ�ʽ��� | |
| D�� | ��ȥSiO2�е�Al2O3�����Թ�����ϡ���ᣬ���ˡ�ϴ�ӡ����T�� |
| A�� | ��ʹ���ȱ�����Һ�У�Na+��Fe2+��SO42-��MnO4- | |
| B�� | $\frac{c��O{H}^{-}��}{c��{H}^{+}��}$=1012����Һ�У�K+��Na+��Cl-��HCO3- | |
| C�� | 0.1 mol•L-1��NH4I��Һ�У�Mg2+��Na+��ClO-��NO3- | |
| D�� | 0.1 mol•L-1��CuSO4��Һ�У�Mg2+��Al3+��Br-��Cl- |
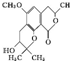
| A�� | ÿ����������2������̼ԭ�� | |
| B�� | ����FeCl3��Һ������ɫ��Ӧ | |
| C�� | 1 mol�����������뺬2 mol NaOH��ˮ��Һ��Ӧ | |
| D�� | ��������3�ֺ��������� |
| A�� | 7.00 | B�� | 1.00 | C�� | 0.7 | D�� | 1.30 |

| A�� | ƻ��֭��c��H+��=0.3mol•L-1 | B�� | ������ˮ��һ��������Һ | ||
| C�� | ʯ��ˮ�ļ��Աȷ���ˮǿ | D�� | ʯ��ˮ��ƻ��֭�ܷ����кͷ�Ӧ |
| A�� | 0.50mol•L-1 | B�� | 0.40mol•L-1 | C�� | 0.20mol•L-1 | D�� | 0.90mol•L-1 |