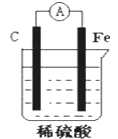
��Ŀ����
����Ŀ����֪��������������ԭ��Ӧ�Ļ�ѧ����ʽ��
��2KMnO4��16HCl(Ũ)===2KCl��2MnCl2��5Cl2����8H2O
��2Al��Fe2O3![]() Al2O3��2Fe
Al2O3��2Fe
��2KClO3![]() 2KCl��3O2��
2KCl��3O2��
��1����Ӧ����������Ϊ________����ԭ��Ϊ________��
��2����Ӧ���и÷�Ӧ��Fe2O3������________��Ӧ��Al������________��Ӧ��
��3����Ӧ����ÿ����1molO2��ת�Ƶ��ӵ����ʵ�����______��
���𰸡�KMnO4 HCl ��ԭ ���� 4mol
��������
��1������Ԫ�ػ��ϼ۽��͵�������������������Ԫ�ػ��ϼ����ߵ������ǻ�ԭ�����ݴ˽��
��2������Al��FeԪ�صĻ��ϼ۱仯�жϣ�
��3��������Ԫ�صĻ��ϼ۱仯���㡣
��1����Ӧ����MnԪ�ػ��ϼ۽��ͣ��õ����ӱ���ԭ��������ΪKMnO4��ClԪ�ػ��ϼ����ߣ�ʧȥ���ӱ���������ԭ��ΪHCl��
��2����Ӧ������Ԫ�ػ��ϼ۽��ͣ��õ����ӣ���÷�Ӧ��Fe2O3�����˻�ԭ��Ӧ����Ԫ�ػ��ϼ����ߣ�ʧȥ���ӣ�Al������������Ӧ��
��3����Ӧ������Ԫ�ػ��ϼ۴ӣ�2�����ߵ�0�ۣ�ʧȥ2�����ӣ���ÿ����1molO2��ת�Ƶ��ӵ����ʵ�����4mol��

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д�����Ŀ���±��Dz�ͬ�¶���ˮ�����ӻ��������Իش��������⣺
�¶�(��) | 25 | t1 | t2 |
ˮ�����ӻ����� | 1��10-14 | �� | 1��10-12 |
(1)��25��t1��t2������______(������������������=��)1��10-14��
(2)25���£�ijNa2SO4��Һ��c(SO42-)=5��10-4mol��L-1��ȡ����Һ1mL����ˮϡ����10mL����ϡ�ͺ���Һ��c(Na+)��c(OH-)=________��
(3)25���£���pH=1��������Һ��pH=5��������Һ�������ϣ�����Һ��ˮ�������c(OH-)=________��
(4)��t2���¶���pH=11�Ŀ�������Һa L��pH=1��ϡ������ҺbL��ϣ����û����ҺpH=2����a��b=______������Һ������Ũ�ȵ��ɴ�С������˳����______________________________��(���Ͼ�������Һ���ǰ������ı仯)
(5)��t2���¶���pH=9�Ŀ�������Һ��pH=y��ϡ������Һ�������ϣ����û����Һ�����ԣ���y____3(������������С��������������)������Һ����Ũ�ȵ��ɴ�С������˳����________________________��(���Ͼ�������Һ���ǰ������ı仯)