��Ŀ����
����Ŀ��FeSO4��7H2O��ҽѧ�ϳ�������Ѫ����ij����С��ͨ����KMnO4�ζ�����õ�FeSO4��Һ���ⶨij��Ѫ����FeSO4�ĺ�����ʵ����������0.01 mol/L 1000 mL��KMnO4��Һ��
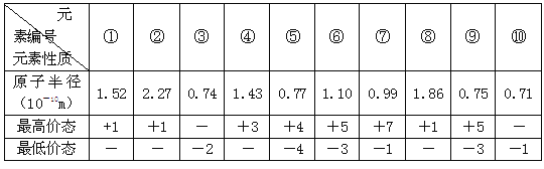
(1)��Ҫ�IJ����������ձ���_______ ��_______ ��_______��
(2)��������ƽ��ȡKMnO4���������Ϊ_______g��
(3)���в���ʹʵ����ƫ����� _______��
A.����ƿδ���� B.δϴ���ձ��Ͳ�����
C.����ʱ��������ƿ�̶��� D.���ձ��ܽ�ʱ��������Һ�彦��
(4)�ֲⶨ��Ѫ����FeSO4�ĺ�����ʵ�鲽�����£�
a.ȡ8Ƭ��Ѫ����Ʒ��ȥ���¡���ĥ���ܽ⡢���ˣ�����Һ���Ƴ�250 mL��Һ
b.ȡ������Һ25 mL����ƿ�У��������������ữ���μ�0.01 mol/L��KMnO4��Һ����ӦΪ10 FeSO4+2KMnO4+8H2SO4=5Fe2(SO4)3 +2MnSO4+K2SO4+8H2O����¼�ζ��յ�ʱ����KMnO4��Һ��������ٶ�ҩƷ�������ɷֲ���KMnO4��Ӧ����
c.�ظ�����b 2-3�Σ�ƽ������KMnO4��Һ20.00 mL��
�ò�Ѫ����FeSO4�ĺ���Ϊ _______ mg/Ƭ��д��������̣�
���𰸡������� ��ͷ�ι� 1000 mL ����ƿ 1.6 C 190 mg
��������
(1)�����������ʵ���Ũ�ȵ���Һ�IJ���ȷ��ʹ�õ�������
(2)����n=cV�������ʵ����ʵ��������m=nM�������ʵ�������
(3)����c=![]() �жϲ�����
�жϲ�����
(4)���ݷ���ʽ10FeSO4+2KMnO4+8H2SO4=5Fe2(SO4)3 +2MnSO4+K2SO4+8H2O��FeSO4��KMnO4�ķ�Ӧ��ϵ������250mL��Һ(��8ƬҩƬ)�к��е�FeSO4�����ʵ����������������ɵ�ÿƬҩƬ�к��е�FeSO4��������
(1)����1000mL0.01mol/L��KMnO4��Һ����Ҫʹ�õ��������ձ�������������ͷ�ιܺ�1000mL������ƿ�Ѿ��������ձ�����˻�ȱ�ٵ������Dz���������ͷ�ιܺ�1000mL������ƿ��
(2)����1000mL0.01mol/L��KMnO4��Һ����Һ�к������ʵ����ʵ���n=cV=0.01mol/L��1L=0.01mol��������������ʵ�����m=nM=0.01mol��158g/mol=1.58g������������ƽ��ȷ����0.1g�����Ҫ������������1.6g��
(3)A.����ƿδ����������ʵ��������䣬����ʱ��Һ�����Ҳ���䣬��˶������Ƶ���Һ��Ũ����Ӱ�죬A���������⣻
B.δϴ���ձ��Ͳ���������ʹ���ʵ�����ƫ�٣�nƫС�������Ƶ���Һ��Ũ��ƫ�ͣ�B���������⣻
C.����ʱ��������ƿ�̶��ߣ���ʹ��Һ�����VƫС�������ʵ���Ũ�ȶ���ʽ��֪�����ƫС������Һ��Ũ��ƫ�ߣ�C�������⣻
D.���ձ��ܽ�ʱ��������Һ�彦������ʹ���ʵ�����ƫ�٣�nƫС�������Ƶ���Һ��Ũ��ƫ�ͣ�D���������⣻
�ʺ���ѡ����C��
(4)���ݷ���ʽ10FeSO4+2KMnO4+8H2SO4=5Fe2(SO4)3 +2MnSO4+K2SO4+8H2O��֪n(FeSO4)=5n(KMnO4)=5��0.01mol/L��0.02L��![]() =0.01mol��m(FeSO4)=0.01mol��152g/mol=1.52g=1520ml����ÿƬҩƬ�к��е�FeSO4��������m(FeSO4)=1520mg��8=190mg��
=0.01mol��m(FeSO4)=0.01mol��152g/mol=1.52g=1520ml����ÿƬҩƬ�к��е�FeSO4��������m(FeSO4)=1520mg��8=190mg��